Abstract
Keywords:Abdominal Ultrasound; Pediatrics; Radiographs; Paradigm Shift; Pneumoperitoneum; Peristalsis; Pathophysiology
Abbreviations: NEC: Necrotizing Enterocolitis; POCUS: Point-Of-Care Ultrasound
Introduction
Necrotizing enterocolitis (NEC) remains a diagnostic challenge in the absence of biomarkers with a disease spectrum ranging from early NEC with subtle non-specific clinical presentation to severe NEC with bowel necrosis and an acute abdomen requiring surgery for disease eradication [1]. Serious long-term complications include short-gut syndrome from intestinal failure to adverse neurodevelopmental outcomes and death [2]. Since the introduction of Bell’s staging for the diagnosis of NEC in early 1970s, plain abdominal radiograph remains the gold standard primary diagnostic imaging tool [1,3]. X-ray is an excellent tool to diagnose pneumoperitoneum - a universally accepted indication for surgical intervention in NEC [1,3]. While X-ray features of pneumatosis intestinalis and / or portal venous gas define the imaging diagnosis of NEC, its limitations due to poor sensitivity in early NEC and significant inter-observer variability are well-recognized. To overcome the obvious lack of information on bowel vascularity, peristalsis, and detection of fluid within the abdominal cavity using X-rays, abdominal ultrasound emerges as a valuable adjunct and it is increasingly being adopted as a point-of-care ultrasound (POCUS) tool by the neonatal intensivist [4-6]. The complementary strengths and limitations of abdominal radiography and ultrasound in NEC diagnosis are summarized in (Table 1), highlighting the rationale for adopting a multimodal imaging approach.
Ultrasound provides a dynamic, real-time, assessment of the bowel wall integrity, motility, and perfusion vital for a comprehensive assessment in evaluating a suspected diseased bowel state. There has been a recent surge in evidence suggesting that bowel ultrasound identifies features such as bowel wall thickening, absent peristalsis, portal venous gas, and complex peritoneal fluid more reliably than radiographs [7-9]. Doppler interrogation of the intestines is crucial to detect restrictive or absent perfusion patterns predictive of disease severity and impending perforation in infants with severe NEC [10]. In this opinion article, we share the synergistic value of using X-ray and ultrasound imaging in improving the diagnostic framework in NEC and also discuss the role of multi-omics to redefine NEC as a syndrome with multiple phenotypes rather than a single disease entity. Radiography remains the most sensitive imaging tool to detect pneumoperitoneum usually associated with advanced NEC, while ultrasound provides superior sensitivity in early NEC, has greater prognostication value, and can be safely implemented at the bedside.
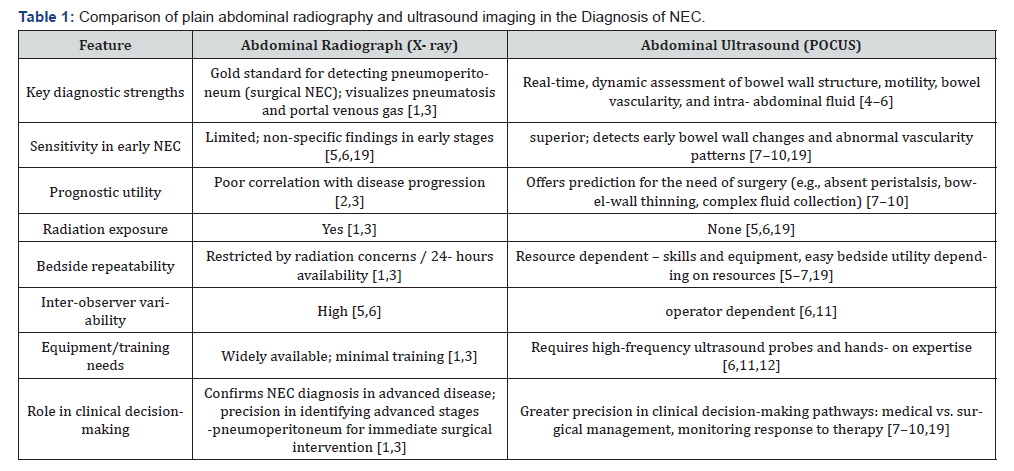
The evolving evidence for utilizing abdominal POCUS in the diagnosis of NEC.
In our scoping review (Diagnostics), we analyzed? exactly how many studies with strong evidence showing that ultrasound outperforms plain radiography in early NEC detection [11]. Additionally, ultrasound findings of absent peristalsis, bowelwall thinning, and complex peritoneal fluid have been correlated with progression to advanced stages of NEC predicting need for surgery [7-10]. Ultrasound is relatively radiation-safe with easy repeatability at the bedside in the hands of a skilled-operator. Thus, ultrasound is a valuable imaging tool in the management of NEC for the dynamic monitoring of disease progression, early detection of feeding intolerance with re-initiation of feeds post therapy to gauge treatment response and recovery [5,6]. The evidence of state-of-the-art findings in NEC have been well described by the radiologists two decades ago, with evolving interest in using abdomen POCUS amongst neonatal clinicians globally. However, this skill has a steep-learning curve. A, availability of high-frequency ultrasound probes and facilitation and implementation of education and training to acquire this skill given the low-incidence of NEC pose challenges in its wider acceptability and routine implementation in clinical practice [6,12]. These challenges have contributed to the slow adoption of ultrasound into routine bedside practice, despite its growing evidence of clinical advantages in NEC diagnosis. In our Frontiers in Pediatrics article, we describe a range of pathological bowel ultrasound findings with clinical correlations, intending to familiarize and orientate neonatal clinicians using point-of-care ultrasound to facilitate the utility of this skill as a diagnostic tool in suspected bowel dysfunction. Ultrasound is particularly valuable when radiographic findings are equivocal- situations that often heighten clinician concern yet provide little guidance in guiding clinical management pathways [12].
Integrating imaging modalities with clinical phenotypic presentations in NEC
Recent diagnostic models illustrate how ultrasound can be pragmatically integrated into neonatal diagnostic pathways. Elsayed and colleagues (202) recently classified NEC based on definite treatment interventions validating the reliability of a predominantly ultrasound integrated framework approach along with clinical and laboratory markers [13,14]. This approach provides diagnostic precision advocating for the ability of ultrasound findings in differentiating various NEC phenotypes. This structured ultrasound evaluation allows for greater diagnostic precision avoiding unnecessary interruptions in feeding, prolonged antibiotic use preventing dysbiosis, which in itself can predispose to NEC [12,15]. (Figure 1) illustrates a proposed integrated diagnostic framework, combining clinical assessment, laboratory data, imaging (radiography and ultrasound), and emerging multi-omics signals to support phenotypic classification of NEC, improving precision in diagnosis and guiding risk stratification to improve outcomes. Surveys of practice variation have highlighted the inconsistencies in NEC diagnosis and treatment addressing the need for standardized decision-making pathways for the management of NEC [12]. Integrated frameworks using multimodal evaluation of X-rays and ultrasound are therefore essential for standardization of clinical care and monitoring outcomes.
Conceptualizing NEC as a Syndrome
Advances in systems biology have challenged us to rethink and redefine the disease pathophysiology of NEC. Josef Neu and colleagues have long argued that NEC is not a single disease but rather a syndrome encompassing several overlapping entities [14]. These include classic preterm NEC, term NEC with ischemic arteriolopathy secondary to hypoxic events or congenital heart disease, food protein-induced enterocolitis. The integration of X-rays with ultrasound provides superior discriminating power to differentiate between the various phenotypes of NEC and NEC-mimics clinically. Radiographs alone are un helpful in distinguishing these different phenotypes of what starts with suspected NEC, a major challenge in clinical management in the absence of a biomarker for this disease [14,15]. Multi-omics research provides further support for this reconceptualization. Genomic studies implicate variants in innate immune regulation, while transcriptomic and proteomic analyses reveal altered expression of barrier and inflammatory mediators. Metabolomic profiling identifies biochemical signatures of ischemia and dysbiosis, while microbiome studies consistently report loss of protective taxa and overrepresentation of Proteobacteria before NEC- onset [15-18]. Together, these findings highlight the multi-factorial etiology in NEC guiding pathways to navigate this information in NEC diagnosis.
Ultrasound as the Phenotypic Anchor
Within the emerging multi-omics framework, ultrasound holds a uniquely valuable role. It offers a dynamic, real-time visualization of intestinal pathophysiology- capturing vital information on bowel-wall structure, motility, perfusion, and fluid dynamics- making it an ideal tool, a phenotypic anchor for multiomics integration. For instance, if the ultrasound reveals sluggish or absent peristalsis, bowel wall thickening, increased vascularity with characteristic NEC- associated patterns, and complex fluid collections [7-10,19]; the metabolomic profiles shows elevated inflammatory mediators and microbiome sequencing indicates a shift toward Proteobacteria dominance, the combined datathough individually insufficient can collectively be helpful to aid in assimilating a NEC profile for the correct diagnosis in the appropriate clinical setting [15-18]. The integration of imaging and multi-omics holds the potential to transform NEC diagnosis from one of uncertainty to one of precision. Recently, combining ultrasound findings with fecal calprotectin have been shown to improve sensitivity and specificity [19]. Longitudinal studies linking microbiome shifts to serial ultrasound findings are beginning to reveal patterns predictive of NEC onset [15,16]. These are early yet impactful steps towards individualized risk stratification pathways, facilitating early targeted interventions, and improving clinical outcomes in NEC [20,21].
Conclusion
Thus, the time has come for a paradigm shift by integrating the synergistic value of bowel ultrasound along with plain X-ray and utilising the advancements in multi-omics to change the diagnostic paradigm of NEC. Abdominal radiography remains a valuable primary screening tool, when integrated with abdominal POCUS, a comprehensive assessment of the bowel including assessment of vascularity, peristalsis, assessment of the intrabdominal cavity can be achieved. Ulti-omics approaches are reshaping our understanding of NEC as a heterogeneous syndrome, and ultrasound is uniquely positioned as the bridge between molecular biology and bedside care.
References
- Bell MJ, Ternberg JL, Feigin RD (1978) Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg 187(1): 1-7.
- Kosloske AM (1994) Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl 396(Suppl 396): 2-7.
- Walsh MC, Kliegman RM (1986) Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33(1): 179-201.
- Faingold R, Daneman A, Tomlinson G (2005) NEC: assessment of bowel viability with color Doppler US. Radiology 235(2): 587-594.
- Bohnhorst B (2013) Usefulness of abdominal US in diagnosing NEC. Arch Dis Child Fetal Neonatal Ed 98(5): F445-F450.
- Alexander KM, Chan SS, Opfer E (2021) Implementation of bowel ultrasound practice for the diagnosis and management of NEC. Arch Dis Child Fetal Neonatal Ed 106(1): 96-103.
- Cuna AC, Reddy N, Robinson AL, Chan SS (2018) Bowel ultrasound for predicting surgical management in NEC: a systematic review and meta-analysis. Pediatr Radiol 48(5): 658-666.
- Cuna AC, Reddy N, Robinson AL (2018) Bowel ultrasound for the diagnosis of NEC: systematic review & meta-analysis. Ultrasound Q 34(3): 113-118.
- Palleri E, Kaiser S, Hall NJ, Eaton S, Wester T, et al. (2017) Complex fluid collection on ultrasound indicates perforation in NEC. Eur J Pediatr Surg 27(2): 161-165.
- Cuna AC, Reddy N, Chan SS (2020) Bowel ultrasound for the management of NEC: a prospective cohort. J Pediatr 225: 58-65.
- Bhattacharjee I, Dolinger MT, Singh R, Singh Y (2025) Ultrasound for the Early Detection and Diagnosis of Necrotizing Enterocolitis: A Scoping Review of Emerging Evidence. Diagnostics 15(15): 1852.
- Priyadarshi A, Sitaula C, He J (2023) Recent advances in neonatal GI point-of-care technologies and the road toward integrated care. Front Pediatr 11: 1173311.
- Elsayed YN, Seshia M (2022) A new intestinal ultrasound-integrated approach for management of neonatal gut injury. Eur J Pediatr 181(4): 1739-1749.
- Neu J (2014) Necrotizing enterocolitis: the mystery goes on. Neonatology 106(4): 289-295.
- Neu J, Pammi M (2017) Pathogenesis of NEC: the role of host-microbial interactions. Semin Perinatol 41(1): 29-35.
- Olm MR, Bhattacharya N, Crits-Christoph A (2019) Necrotizing enterocolitis is preceded by increased gut bacterial replication. Sci Adv 5(12): eaax5727.
- Stewart CJ, Embleton ND, Marrs EC (2016) Longitudinal development of the gut microbiome and metabolome in preterm infants; relationship to NEC. Microbiome 4: 31.
- Niño DF, Sodhi CP, Hackam DJ (2016) Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 13(10): 590-600.
- van Druten J, Khashu M, Chan SS, Sharif S, Abdalla H, et al. (2019) Abdominal ultrasound should become part of standard care for early diagnosis and management of NEC: a narrative review. Arch Dis Child Fetal Neonatal Ed 104(5): F551-F559.
- Alsaied A, Islam N, Thalib L (2020) Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr 20(1): 344.
- Ng PC, Ang IL, Chiu RWK (2010) Host-response biomarkers for diagnosis of late-onset septicemia and NEC in preterm infants. J Clin Invest 120(8): 2989-3000.






























