Abstract
Background: Ventilation management in the immediate post-operative period following duodenal atresia repair in neonates has not been well studied. We report on two cases of postoperative pneumoperitoneum following duodenal atresia (DA) repair in neonates. In both cases, the use of noninvasive ventilation (NIV) was associated with the complication of pneumoperitoneum.
Case Presentation: The first neonate was born preterm at 31 weeks gestation with Trisomy 21, complete atrioventricular septal defect (AVSD), and duodenal atresia. He was intubated for DA repair and was extubated and placed on noninvasive neurally adjusted ventilatory assist (NI-NAVA) on postoperative day two. On postoperative day four, he developed abdominal distention and hypoxemia. Abdominal imaging showed a pneumoperitoneum. Exploratory laparotomy was performed to repair a leak at the anastomotic site. The second case was a term infant with duodenal atresia, myelomeningocele, and other dysmorphic features. She was extubated to high flow nasal cannula on the post-operative day three. Due to increasing respiratory distress, support was escalated to continuous positive airway pressure (CPAP) and then to noninvasive positive pressure ventilation (NIPPV) within a few hours. Increasing abdominal distention led to imaging and showed a pneumoperitoneum. A leak at the anastomotic site was identified and repaired.
Conclusion: The post-operative complication of anastomotic leak is a rare complication of DA repair. The two cases described above demonstrate that post-operative NIV could potentially lead to air distention of the stomach and duodenum and may contribute to a leak at the anastomotic site. To our knowledge, this complication of DA repair has not been previously reported in the literature and should be considered when extubating patients to NIV in the immediate postoperative period.
Keywords: Duodenal Atresia; Continuous Positive Airway Pressure; Non-Invasive Ventilation; High Flow Nasal Cannula
Abbreviations: DA: Duodenal Atresia; CPAP: Continuous Positive Airway Pressure; NIV: Non-Invasive Ventilation; HFNC: High Flow Nasal Cannula; AVSD: Atrioventricular Septal Defect; NI-NAVA: Noninvasive Neurally Adjusted Ventilatory Assist; NIPPV: Noninvasive Positive Pressure Ventilation
Introduction
Duodenal atresia is a congenital obstruction of the duodenum, usually occurring just past the ampulla of Vater, seen in 1 in 5,000-10,000 births [1,2]. It may be associated with polyhydramnios. Affected newborns often present after birth with abdominal distention and vomiting (bilious or non-bilious) within 1-2 days.
Noninvasive ventilation (NIV) methods like high flow cannula, CPAP, and NIPPV are commonly used to support neonates after extubation, but safety of these modes of ventilation in post-duodenal atresia cases is unclear. NIV may increase the risk of gastrointestinal air distention and complications such as anastomotic leak or pneumoperitoneum in the immediate postoperative period. Recognizing and addressing these risks early is vital for optimal outcomes. Duodenal atresia can occur as an isolated condition or in association with other malformations or syndromes like Trisomy 21. Diagnosis can be made by a plain abdominal x-ray, which will often show a double bubble sign. A limited upper GI study can confirm the diagnosis. Surgical repair can be performed either by open or laparoscopic hand-sewn techniques. Both methods have an excellent prognosis, with no significant difference in complication rates, including anastomotic leaks [3-5]. Postoperative complications from NIV are rare, though pneumoperitoneum has been reported. [4] Re-exploration increases morbidity, leading to prolonged hospital stay, extended parenteral nutrition, sepsis, and parental dissatisfaction.
Case Reports
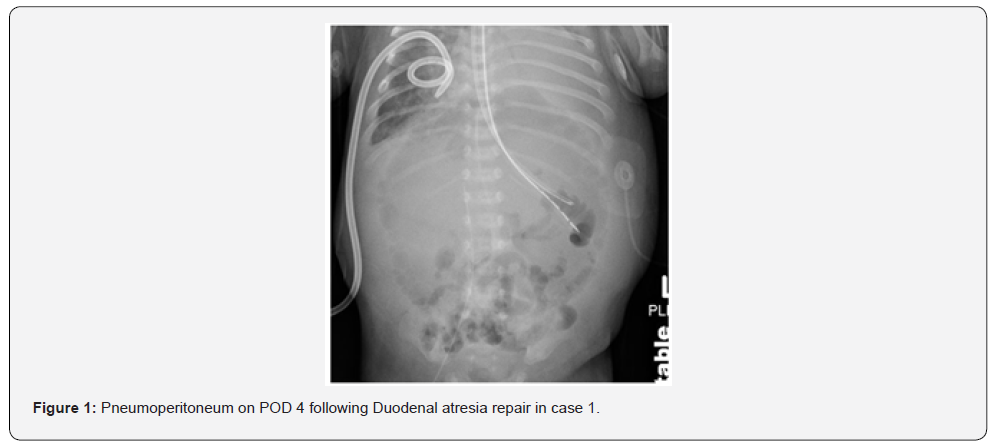
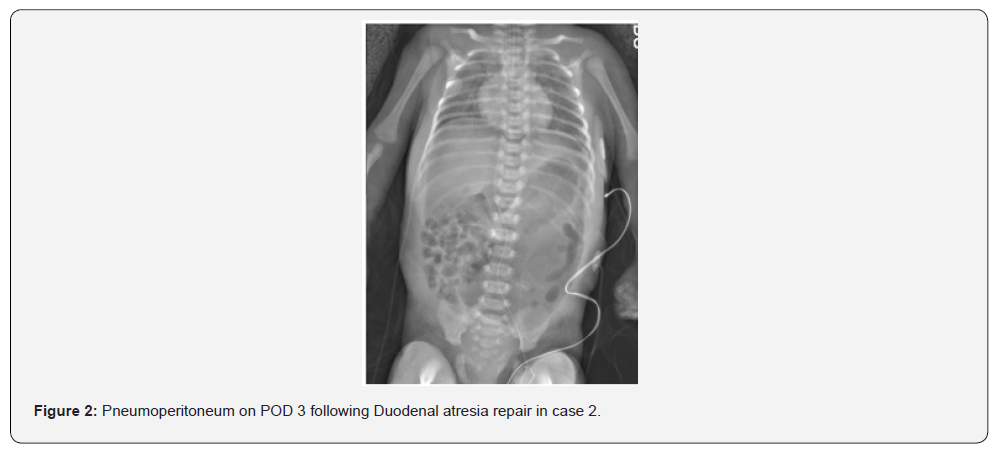
The first neonate, born at 31 weeks gestation with Trisomy 21, complete AVSD, pleural effusion, and duodenal atresia, was preoperatively on NI-NAVA. He was intubated for duodenal atresia repair and extubated to NI-NAVA on postoperative day two. On post-operative day four, he developed abdominal distention and hypoxemia; imaging revealed pneumoperitoneum (Figure 1). During laparotomy, worsening distention and hypoventilation prompted emergency abdominal drainage of gas and serous fluid. He required two doses of epinephrine and chest compressions briefly for bradycardia. A small anterior wall leak was repaired. Two months later, he underwent cardiac surgery and was eventually discharged home at six months of age. The second neonate, born at 37 weeks gestation and small for her gestational age, with duodenal atresia, myelomeningocele, and musculoskeletal deformities, underwent duodenal atresia repair where an annular pancreas was found. She was initially extubated to high flow nasal cannula, but within hours she required escalation of support to CPAP and then NIPPV. Seven hours post-extubation, she developed abdominal distension; imaging showed pneumoperitoneum (Figure 2). Re-exploration revealed a leaking anastomosis due to suture dehiscence, which was then repaired. She was successfully extubated to room air on postoperative day five and tolerated feeds.
Discussion
This report discusses the critical role of post-operative ventilation management in neonates after intestinal surgeries, especially foregut procedures with primary anastomoses. Noninvasive ventilation (NIV) can cause high pressure and airflow to the esophagus, stomach, and duodenum, increasing the risk of complications like anastomotic dehiscence and leaks. Excess airflow to a distended proximal segment, combined with reduced flow through a narrowed distal segment, may place additional stress on a new anastomosis. In the cases described above, causation cannot be proven but temporal association of complications with NIV signifies association. Escalation in the NIV support was seen prior to the onset of symptoms in the second case. It is likely that excess airflow to the distended proximal segment with decreased airflow through the distal relatively narrow segment leads to increased stress on the fresh anastomotic site. Factors like gestational age, pre-existing associated anomalies, and degree of positive pressure ventilation via NIV affect outcomes.
Early extubation is favored in the NICU due to its links to shorter stays, fewer ventilation-related lung injuries in non-surgical infants, and lower costs. Non-invasive ventilation (NIV) lowers extubation failure rates but may raise the risk of anastomotic leaks when used after foregut repair surgery. Prolonged opiate exposure and lower gestational age increase the need for ongoing ventilatory support postoperatively. Positive pressure from NIV can lead to leakage or disruption at the anastomotic site [6,7]. Anastomotic leaks are a common complication after esophageal atresia repair, potentially causing mediastinitis, sepsis, pneumothorax, or pleural effusion [8-11]. In a retrospective study by Chittmittrapap et al, 199 neonates underwent primary or delayed primary repair of esophageal atresia, 34 (17%) of infants developed anastomotic leakage, 7 (3.5%) had major anastomotic disruptions [12].
Literature on risk factors for anastomotic leak following duodenal atresia is scarce because of the rarity of the complication. In a retrospective case series by Kulkarni et al, a high rate of complication (sepsis, anastomotic leak) and mortality was noted in low-birth-weight infants with duodenal atresia repair, however, correlation of complications with ventilatory support was not evaluated [13]. In the post-operative period, the presence of systemic signs of sepsis, significant abdominal tenderness or abdominal wall erythema should raise the suspicion of anastomotic leak. Pneumoperitoneum can occur after surgical repair due to insufflation, anastomotic leak, or visceral perforation [14]. Risk factors in neonates include primary diagnosis, gestational age, weight at surgery, duration of opioids administration, and noninvasive ventilation [15]. Significant pneumoperitoneum on a plain abdominal radiography is sufficient to make the diagnosis of anastomotic leak. Urgent re-exploration in the operating room is needed to address the cause of the anastomotic leak, with repair options include suture reinforcement or repeat anastomosis in case of dehiscence. Placement of a closed suction drain in the operative area is important [16,17]. The postoperative mortality rate in duodenal atresia is reported to be 4% to 5%. [18]. The mortality rate is higher in patients with trisomy 21 and other associated congenital anomalies, especially in those with cardiac defects.
In summary, post-operative pneumoperitoneum following duodenal repair is an uncommon occurrence and has not been well documented in medical literature. The post-operative goal for ventilation is early extubation to avoid lung injury associated with prolonged invasive ventilation. However, premature extubation can increase the need for NIV support and distention of the gastrointestinal tract leading to anastomotic leaks presenting with pneumoperitoneum. Therefore, cautious use of non-invasive ventilation may prevent this post-operative complication in infants with repaired duodenal atresia.
Patient Consent: Informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images.
References
- Cruz-Centeno N, Stewart S, Marlor DR, Aguayo P, Rentea RM, et al. (2023) Duodenal Atresia Repair: A Single-Center Comparative Study. Am Surg 89(12): 5911-5914.
- Moga A, Bălănescu R, Bălănescu L, Cîmpeanu P, Andriescu M, et al. (2024) Factors Associated with Postoperative Complications After Congenital Duodenal Obstruction Surgery: A Retrospective Study. Medicina (Kaunas) 60(10): 1722.
- Piper HG, Alesbury J, Waterford SD, Zurakowski D, Jaksic T, et al. (2008) Intestinal atresia’s: factors affecting clinical outcomes. J Pediatr Surg 43(7): 1244-1248.
- Sarin YK, Sharma A, Sinha S, Deshpande VP (2012) Duodenal Web. An experience with eighteen patients. Journal of Neonatal surgery 1(2): 20.
- Martou L, Saxena AK (2024) Laparoscopic repair of duodenal atresia: systematic review and meta-analysis after consistent implementation of the technique in the past decade. Meta-Analysis 38(6): 3296-3309.
- Ferrand A, Roy S, Aspirate A, Faure C (2017) Post Operative Non-Invasive Ventilation and Complications in Esophageal Atresia-Tracheoesophageal Fistula. Pediatric Child Health 22(Suppl1): e25.
- Wang H, Gauda EB, Chiu PL, Moore AM (2022) Risk Factors for Prolonged Mechanical Ventilation in Neonates Following Gastrointestinal Surgery. Transl Pediatric 11(5): 617-624.
- Ferrand A, Roy SK, Faure C (2019) Post Operative Non-invasive Ventilation and complications in Esophageal Atresia-tracheoesophageal Fistula. J Pediatr Surg 54: 945-948.
- Morini F, Conforti A, Bagolan P (2018) Perioperative Complications of Esophageal Atresia. Eur J Pediatr Surg 28(2): 133-140.
- Zhao R, Li K, Shen C (2011) The outcome of conservative treatment for anastomotic leakage after surgical repair of esophageal atresia. J Pediatr Surg 46(12): 2274-2278.
- Askarpour S, Peyvasteh M, Javaherizadeh H (2025) Evaluation of risk factors affecting anastomotic leakage after repair of esophageal atresia. Arq Bras Cir Dig 28(3): 161-162.
- Chittmittrapap S, Spitz L, Kiely EM, Brereton RJ (1992) Anastomotic leakage following surgery for esophageal atresia. J Pediatr Surg 27(1): 29-32.
- Kulkarni A, Gupta A, Kothari P, Jayaswal S, Dikshit V, et al. (2019) Retrocolic Isoperistaltic Gastrojejunostomy as an Alternative to Kimura's Duodenoduodenostomy in Low- and Very Low-birth-weight Babies of Duodenal Atresia: A 5 Year Retrospective Study. Journal of Clinical Neonatology 8(2): 75-78.
- Antabak A, Bogović M, Vuković J, Grizelj R, Babić VB, et al. (2016) Postoperative gastric perforation in a newborn with duodenal atresia. J Neonate Surg 5(4): 62.
- Kähler G (2017) Anastomotic Leakage after Upper Gastrointestinal Surgery: Endoscopic Treatment. Visc Med 33(3): 202-206.
- Burjonrappa S, Crete E, Bouchard S (2011) Comparative outcomes in intestinal atresia: a clinical outcome and pathophysiology analysis. Pediatric Surg Int 27(4): 437-442.
- Zhang Q, Chen Y, Hou D, Guo W (2005) Analysis of postoperative reoperation for congenital duodenal obstruction. Asian J Surg 28(1): 38-40.
- Grosfeld JL, Rescorla FJ (1993) Duodenal atresia and stenosis: Reassessment of treatment and outcome based on antenatal diagnosis, pathologic variance, and long-term follow-up. World J Surg 17(3):301-309.






























