Introduction
Obesity constitutes one of the most frequent reasons for consultation in clinical practice, both in adults and in the paediatric field. The susceptibility of each patient to the development of obesity, expressed as an excessive accumulation of adipose tissue as a phenotypic manifestation common to different conditions, environments and under the determinants of a marked genotype. In recent years, the pathophysiological basis of appetite control, satiety and energy expenditure has been clarified. The underlying genetic, non-genetic and endocrinological causes and conditions have also been identified. Among the advances in the study of aetiologies, a growing number of so-called monogenic aetiologies have been characterised in recent years, thanks to increased knowledge of the pathophysiological basis of obesity. The causes of monogenic obesities preferentially affect genes involved in the appetite-satiety regulation of the leptin-melanocortin pathway: leptin (LEP), leptin receptor (LEPR), proopiomelanocortin (POMC), proprotein convertase subtilisin-kexin-like proprotein convertase 1 (PCSK1), melanocortin receptor 4 (MC4R), Src-homology-2B adaptor protein 1 (SH2B1), steroid receptor coactivator 1 (SRC1), carboxypeptidase E (CPE), and steroid receptor coactivator 1 (SRC1). and G protein alpha activity- stimulating polypeptide 1 (GNAS). Of particular interest are the reviews carried out by Martos-Moreno [1,2] and collaborators in this regard, as well as highlighting some widely studied syndromic entities in paediatrics, such as Bardet-Biedl and Alstrom syndromes. It is therefore necessary to thoroughly investigate the cause of a patient’s obesity in order to be able, if necessary, to individualise the therapeutic approach: from the well-known hygiene and dietary measures to pharmacological advances, such as recombinant leptin, the melanocortin analogue setmelanotide and GLP-1 analogues. We present the case of a boy currently 11 years old with monogenic obesity presenting a pathogenic variant in heterozygous variation in the ADCY3 gene (600291) on chromosome 2p23. There are only 10 families described in the literature.
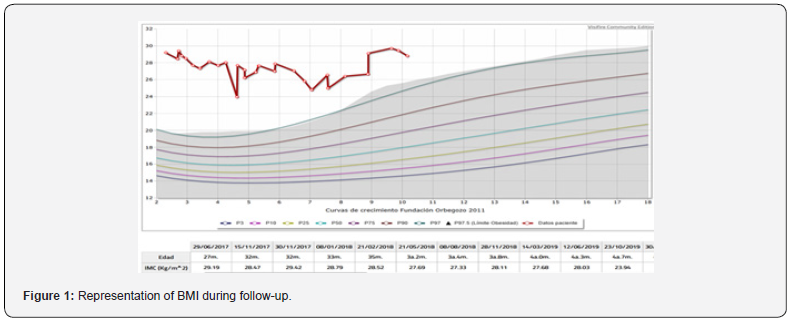
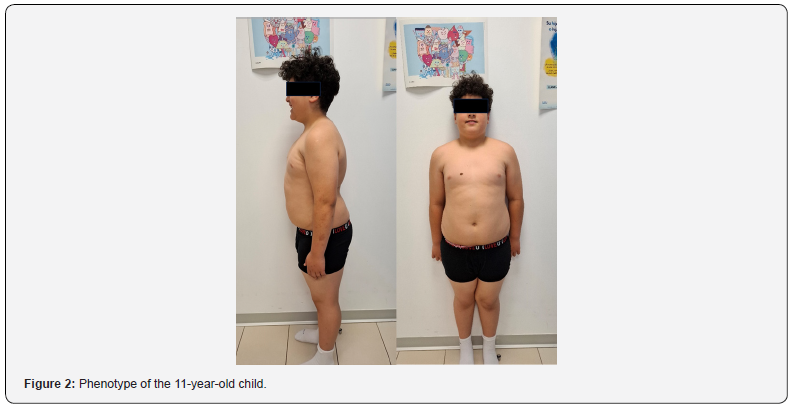
This is a child being followed up at the paediatric endocrinology clinic since the age of 8 months of age for presenting a high height and weight gain well above height, which became more evident after the introduction of the child’s family originated in Morocco. The family origin is Moroccan. There is no consanguinity. The parents are healthy, and the siblings are not affected by significant obesity. The pregnancy was normal and the somatometry at birth for 39 weeks of gestation was adequate: weight of 3300 grams (+0.85 SDS) and height of 50 cm (+0.3 SDS). The physical examination at the first consultation already drew attention with a somatometry at 8 months of life of Weight: 17.9kg (>2 SDS) Height: 81.9cm (>2 SDS) and BMI: 26.6 Kg/m2 (+ 5.5 SDS) Body surface area: 0.6 m2. The phenotype is peculiar, cushingoid in appearance. Good general condition. No rashes or petechiae, no skin patches, no palpable goitre. Pubarche I Penis buried in fat Gonads prepubertal. Exclusive breastfeeding until 5 months of age, after which conventional complementary feeding is introduced, with progressive increase in weight/height. He referred to the clinic for primary care studies. We completed basic studies without finding any altered parameters. Intensive followup with hygienic-dietary measures was initiated. No success over the years (Figure 1) in containing the BMI. Pituitary function and thyroid function are preserved. The cushingoid phenotype is maintained. In addition, she is under multidisciplinary followup for mild intellectual disability, language disorder (Figure 2) (parental consent for publication of photos). Given the suspicion of a syndrome, a karyotype and X-fragment study was performed and was negative. However, given the high clinical suspicion, in 2023 an extension of the genetic study was requested at the external reference laboratory qGenomics. A massive sequencing of the exome was performed, detecting the heterozygous nonsense variant in the ADCY3 gene compatible with monogenic obesity type OMMIN 617885. Moreover, the segregation study in parental samples has shown that the variant originated de novo.
Patients with biallelic mutations in the ADCY3 gene show hyperphagia within the first 2 years of life and develop severe obesity. Other features include hyposmia or anosmia, and some patients exhibit mild to moderate intellectual disability [3]. Reported 4 children with severe early-onset obesity from 3 consanguineous Pakistani families, as well as an obese boy from a European American family. Hyperphagia in the probands was first reported between 6 months and 2 years of age. Other features included anosmia in 3 patients and hyposmia in 2, and 2 probands exhibited mild to moderate intellectual disability. In addition, the oldest patient, a 15-year-old Pakistani girl, had undergone menarche at age 14 years but had no subsequent menstrual cycles; she also exhibited hyperlipidemia and insulin resistance. No dysmorphic features were observed in the patients. Family members were all normal weight, except for the parents of 1 of the Pakistani patients (family 2), who were upper-middle class and accustomed to fat- and carbohydrate-enriched food. Stergiakouli et al. [4] performed genomewide association studies involving BMI and height-adjusted BMI (‘BMI[x]’) in a British and a Dutch cohort of children (ALSPAC and Generation R, respectively). A missense SNP in the ADCY3 gene (rs11676272) showed evidence of genomewide significance in the ASPAC cohort (p = 4 x 10(-9)), but only when height was adjusted for, and this association was replicated in the Generation R cohort (p = 0.0001). Analysis of ADCY3 expression in lymphoblastoid cell lines showed a strong association between the rare (adiposity-increasing) G allele at rs11676272 and reduced levels of ADCY3 expression (p = 1 x 10(-53)). The authors concluded that rs11676272 represents a functional polymorphism in ADCY3 that is associated with fat mass in childhood and noted that the effect size is comparable to common variation at the FTO locus (610966), but only when height is correctly considered. Saeed et al. [3] characterized the rs11676272 (S107P) variant in BHK cells and observed that the mutant showed catalytic activity comparable to that of wildtype ADCY3.
Grarup et al. [5] identified a splice site variant in the ADCY3 gene (600291.0001) with a minor allele frequency of 2.3% in a Greenlandic study population of 4,038 individuals. The 7 homozygous carriers had BMIs, body fat percentages, and waist circumferences that were all significantly greater than those of the remaining study population, and an association with type 2 diabetes (T2D) was found that remained significant after adjustment for BMI. Homozygous carriers showed severely decreased RNA expression, whereas heterozygotes had an intermediate expression level. In addition, analysis of exome-sequencing data from 18,176 samples from the Accelerating Medicines Partnership Type 2 Diabetes Knowledge Portal (AMP-T2D) database identified heterozygosity for 7 predicted loss-of-function variants in ADCY3 in 8 individuals, and there was enrichment of carriers of ADCY3 variants among T2D cases compared to nondiabetic controls (odds ratio, 8.6; p = 0.044). In 138 probands with severe early-onset obesity from consanguineous Pakistani families, Saeed et al. (2018) performed whole-exome sequencing, and in 4 severely obese children from 3 families, they identified homozygous mutations in the ADCY3 gene (see, e.g., 600291.0002 and 600291.0003). Independent wholeexome sequencing in an obese boy from a nonconsanguineous European American family revealed compound heterozygosity for mutations in ADCY3 (600291.0004 and 600291.0005). All mutations occurred at highly conserved sites, and all segregated with disease in the respective families.
Literature
A number sign (#) is used with this entry because of evidence that susceptibility to obesity is conferred by homozygous or compound heterozygous variation in the ADCY3 gene (600291) on chromosome 2p23. For a phenotypic description and a discussion of genetic heterogeneity of body mass index (BMI), see 606641.
Description
Patients with biallelic mutations in the ADCY3 gene show hyperphagia within the first 2 years of life and develop severe obesity. Other features include hyposmia or anosmia, and some patients exhibit mild to moderate intellectual disability [3].
Clinical Features
Saeed et al. [3] reported 4 children with severe early-onset obesity from 3 consanguineous Pakistani families, as well as an obese boy from a European American family. Hyperphagia in the probands was first reported between 6 months and 2 years of age. Other features included anosmia in 3 patients and hyposmia in 2, and 2 probands exhibited mild to moderate intellectual disability. In addition, the oldest patient, a 15-year-old Pakistani girl, had undergone menarche at age 14 years but had no subsequent menstrual cycles; she also exhibited hyperlipidemia and insulin resistance. No dysmorphic features were observed in the patients. Family members were all normal weight, except for the parents of 1 of the Pakistani patients (family 2), who were upper-middle class and accustomed to fat- and carbohydrate-enriched food.
Molecular Genetics
Stergiakouli et al. [4] performed genomewide association studies involving BMI and height-adjusted BMI (‘BMI[x]’) in a British and a Dutch cohort of children (ALSPAC and Generation R, respectively). A missense SNP in the ADCY3 gene (rs11676272) showed evidence of genomewide significance in the ASPAC cohort (p = 4 x 10(-9)), but only when height was adjusted for, and this association was replicated in the Generation R cohort (p = 0.0001). Analysis of ADCY3 expression in lymphoblastoid cell lines showed a strong association between the rare (adiposityincreasing) G allele at rs11676272 and reduced levels of ADCY3 expression (p = 1 x 10(-53)). The authors concluded that rs11676272 represents a functional polymorphism in ADCY3 that is associated with fat mass in childhood and noted that the effect size is comparable to common variation at the FTO locus (610966), but only when height is correctly taken into account. Saeed et al. [3] characterized the rs11676272 (S107P) variant in BHK cells and observed that the mutant showed catalytic activity comparable to that of wildtype ADCY3. Grarup et al. [5] identified a splice site variant in the ADCY3 gene (600291.0001) with a minor allele frequency of 2.3% in a Greenlandic study population of 4,038 individuals. The 7 homozygous carriers had BMIs, body fat percentages, and waist circumferences that were all significantly greater than those of the remaining study population, and an association with type 2 diabetes (T2D) was found that remained significant after adjustment for BMI. Homozygous carriers showed severely decreased RNA expression, whereas heterozygotes had an intermediate expression level. In addition, analysis of exome-sequencing data from 18,176 samples from the Accelerating Medicines Partnership Type 2 Diabetes Knowledge Portal (AMP-T2D) database identified heterozygosity for 7 predicted loss-of-function variants in ADCY3 in 8 individuals, and there was enrichment of carriers of ADCY3 variants among T2D cases compared to nondiabetic controls (odds ratio, 8.6; p = 0.044). In 138 probands with severe early-onset obesity from consanguineous Pakistani families, Saeed et al. [3] performed whole-exome sequencing, and in 4 severely obese children from 3 families, they identified homozygous mutations in the ADCY3 gene (see, e.g., 600291.0002 and 600291.0003). Independent wholeexome sequencing in an obese boy from a nonconsanguineous European American family revealed compound heterozygosity for mutations in ADCY3 (600291.0004 and 600291.0005). All mutations occurred at highly conserved sites, and all segregated with disease in the respective families.
References
- GA Martos-Moreno J (2022) Argente Obesidades monogénicas en la infancia: hacia una medicina de precisión Rev Esp Endocrinol Pediatr 13(Suppl 2): 23-32.
- GA Martos-Moreno J (2011) Argente Obesidades pediátricas: de la lactancia a la adolescencia. An Pediatr (Barc) 75(1): 63.e1-63.e23.
- Saeed S, Bonnefond A, Tamanini F, Mirza MU, Manzoor J, et al. (2018) Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nature Genet 50(2): 175-179.
- Stergiakouli E, Gaillard R, Tavare JM, Balthasar N, Loos RJ, et al. (2014) Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity 22(10): 2252-2259.
- Grarup N, Moltke I, Andersen MK, Dalby M, Vitting-Seerup K, et al. (2018) Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nature Genet 50(2): 172-174.






























