Rivaroxaban for the Treatment of Venous Thromboembolism in NPHS1 Congenital Nephrotic Syndrome
Saraiva BS1, Alves EC1, Sousa A1, Borges MA1, Costa MS-M1, Baptista RB1, Batalha S2, Maia R2, Neto G1, Kjöllerström P2 and Francisco T1
1Paediatric Nephrology Unit, Hospital Dona Estefânia, Unidade Local de Saúde de São José, Academic Clinical Centre Lisbon, Lisbon, Portugal
2Paediatric Haematology Unit, Hospital Dona Estefânia, Unidade Local de Saúde de São José, Academic Clinical Centre Lisbon, Lisbon, Portugal
Submission: May 09, 2024;Published: June 05, 2024
*Corresponding author: Bárbara Martins Saraiva, Paediatric Nephrology Unit, Hospital Dona Estefânia, Unidade Local de Saúde de São José, Academic Clinical Centre Lisbon, Lisbon, Portugal. Rua Jacinta Marto 1169-045 Lisbon, Portugal. Email: barbaracsaraiva@campus.ul.pt; barbara.saraiva@ulssjose.min-saude.pt
How to cite this article: Saraiva BS1, Alves EC1, Sousa A1, Borges MA1, Costa MS-M, et al. Rivaroxaban for the Treatment of Venous Thromboembolism in NPHS1 Congenital Nephrotic Syndrome. Acad J Ped Neonatol 2024; 14(1): 555934. 10.19080/AJPN.2024.14.555934
Abstract
Introduction: Nephrotic syndrome (NS) is associated with a multifactorial hypercoagulable state. Congenital NS (CNS) exhibits a higher prevalence of thrombotic events compared to other types. Direct oral anticoagulants (DOAC) have been approved for paediatric acute venous thromboembolism.
Case Report: We present 2 CNS children treated with rivaroxaban for treatment (Case 1) and prophylaxis (Case 2) of thrombotic events. A 2-month-old male previously diagnosed with CNS with homozygous mutation in the NPHS1 gene, underwent central venous catheter (CVC) replacement during which multiple thrombi were seen. The patient developed clinical signs compatible with pulmonary embolism. Chest radiograph showed a peripheral condensation on the left hemithorax and CT-angiography was inconclusive for peripheral embolism. Despite therapeutic doses of enoxaparin, adjustments were difficult with persistently low anti-Xa levels. The switch to rivaroxaban was performed, and doses were regularly adjusted based on patient’s weight. No adverse or other thrombotic events were reported, despite maintaining CVC. As expected, chronic kidney disease progressed at 19 months and rivaroxaban was suspended. An 8-month-old female with CNS associated with an heterozygous mutations in the NHPS1 gene, underwent multiple CVC replacements due to recurrent obstruction despite heparinisation and alteplase administrations. Although there were no systemic thrombotic episodes, considering the high risk of thrombosis, prophylaxis with rivaroxaban was initiated with eGFR of 54 mL/min/1.73m2 (1-2 SD below expected eGFR). Weight-adjusted dose was prescribed. No severe adverse or thrombotic events reported until 19 months.
Conclusion: These cases suggest that the safety and efficacy profile of rivaroxaban may be encouraging for treating and preventing venous thromboembolism in CNS. However, additional studies are warranted to optimize DOAC use in children with complex conditions, such as CNS, allowing for more tailored management of anticoagulation in this high-risk population.
Keywords: Congenital nephrotic syndrome; NPHS1; Rivaroxaban; Thromboembolism
Abbreviations: CNS: Congenital Nephrotic Syndrome; CVC: Central Venous Catheter; eGFR: estimated Glomerular Filtration Rate; SD: Standard Deviations; NS: Nephrotic Syndrome; DOAC: Direct Oral Anticoagulants
Case Report
Case 1
A four-week-old male infant presented with generalized oedema, abdominal distention, vomiting, and irritability. He was the first child of a non-consanguineous couple and his family history was unremarkable. Prenatally, the ultrasound performed at 36 weeks of gestation revealed oligohydramnios. At the 38-week delivery, APGAR was 9/10 at the 1st and 5th minute respectively. The weight of the placenta was not recorded. No dysmorphic features were described. He was diagnosed with congenital nephrotic syndrome (CNS) and subsequent genetic testing revealed a homozygous mutation in the NPHS1 gene.
The patient received albumin and furosemide as needed according to clinical evaluation. A central venous catheter (CVC) was placed because of the need for frequent albumin administrations. Therapy included dipyridamole, captopril, indomethacin, and levothyroxine, as well as magnesium, calcium, and vitamin D supplementation.
At 2 months, the patient underwent CVC replacement due to tip displacement. During the procedure, several thrombi were identified. Serum albumin (10.1 g/L) and haemoglobin (8.4 g/dL) levels prompted the administration of albumin and packed red blood cells transfusion. In the first 24 hours after CVC substitution, the patient exhibited acute pallor, tachycardia, shortness of breath, and hypoxemia, with no fever. A presumptive diagnosis of peripheral pulmonary embolism was assumed. Chest radiograph showed a peripheral condensation on the left hemithorax. Computed tomography angiography scan ruled out a major pulmonary embolism but provided inconclusive results regarding peripheral embolic events.
Prophylactic dipyridamole was suspended, and subcutaneous enoxaparin was started at therapeutic dose (1.5 mg/kg/dose every 12 hours) according to the age group. Dose adjustments were difficult with persistently low anti-Xa levels. Additional thrombotic risk factors were analyzed (Table 1). The switch to rivaroxaban was performed after 11 days of enoxaparin. At that time, his weight was 4 Kg, with an estimated glomerular filtration rate (eGFR) of 94 mL/min/1.73 m2. The patient received 1.4 mg of rivaroxaban, every 8 hours. As expected, chronic kidney disease progressed reaching an eGFR of 22 mL/min/1.73 m2 (≥ 2 standard deviations (SD) below normal eGFR) at the age of 19 months and rivaroxaban was suspended (1.5 years after starting treatment) as there were no other reported thrombotic events. During treatment, doses were adjusted regularly based on patient’s weight, no other thrombotic events were reported despite maintaining a CVC, and no adverse effects were described.
Case 2
A five-week-old female infant was admitted with failure to thrive, anorexia, prostration and generalized oedema. No family history was reported, and parents were non-consanguineous. During pregnancy, a 32-weeks ultrasound showed foetal growth restriction. Delivery occurred at 34 weeks, with APGAR scores of 8 and 10 at 1st minute and 5th minute, respectively. An enlarged placenta was described. No dysmorphic features were reported. The patient was diagnosed with CNS and later found to have two heterozygous mutations in the NPHS1 gene. Albumin infusions, furosemide, prophylactic dipyridamole, captopril, levothyroxine, iron, magnesium, calcium, and vitamin D supplementation were started upon diagnosis.
In the first 8 months, five CVCs were replaced due to obstruction despite regular heparinisation and alteplase administrations as needed. In each procedure, antithrombin III was administered prophylactically (50 U/Kg). No systemic thrombotic episodes were identified and the doppler ultrasound of neck vessels was normal. Additional coagulation and thrombotic risk evaluations were performed (Table 1).
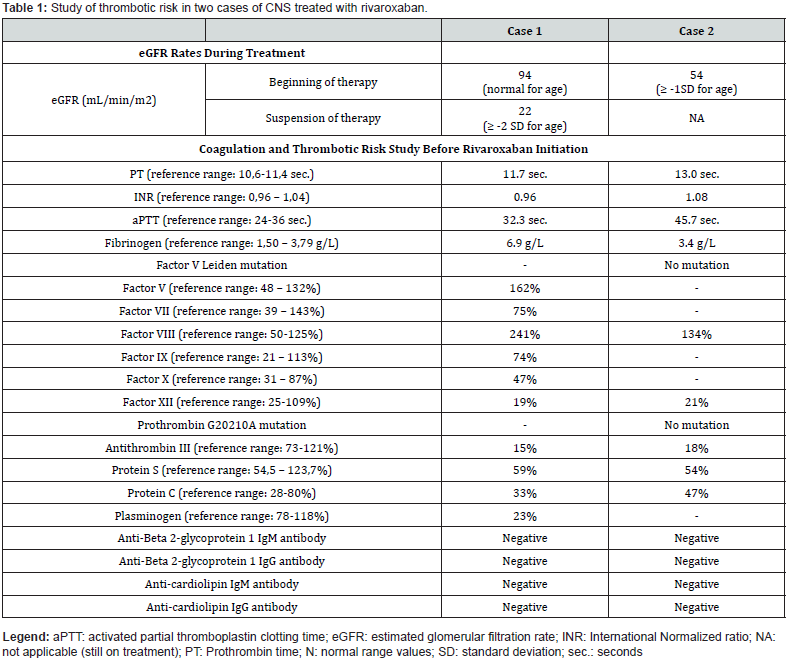
Due to high thrombotic risk and CVC obstructions, rivaroxaban started at 8 months of age. Body weight was 5,7 Kg and eGFR was 54 mL/min/1.73 m2 (1 to 2 SD below normal eGFR) at that time. Weight-adjusted starting dose was 1.6 mg, every 8 hours. Spontaneous self-limited epistaxis was reported on the second day of treatment. No other adverse events or haemorrhagic episodes were described.
Currently, at 19 months, weighing 8.4 Kg and with an eGFR of 53 ml/min/1.73 m2, the patient still has a CVC in place and treatment with rivaroxaban has been maintained, at a present dose of 2.4 mg, every 8 hours according to guidelines. Since the initiation of rivaroxaban, there was only one readmission due to CVC obstruction, after 9 months of therapy.
Discussion
The hypercoagulable state in nephrotic syndrome (NS) is multifactorial and well established. The disruption of the glomerular basement membrane causes the loss of anticoagulants (antithrombin III, plasminogen, protein C, and protein S), along with low molecular weight procoagulants. As a response, there is a hepatic overproduction of high molecular weight procoagulant factors, including fibrinogen and factors V, VII, VIII and X. The leakage of megakaryopoiesis inhibitors promotes dysregulation of platelet production and activation. Therefore, this dysregulation and loss of anticoagulants, like antithrombin III, might explain the inefficacy of low molecular weight heparins in NS [1,2].
In CNS, there is a synergic action of the hypercoagulability of NS along with other pro-thrombotic factors concerning therapy (presence of a CVC, use of diuretics) and hydroelectrolytic disorders (hypocalcaemia, hypomagnesemia) [2]. Severe hypoalbuminemia and intravascular volume depletion (or hypovolaemia) also contribute to this risk. Higher incidence of thromboembolic events has been shown in congenital cases [2] and a recent meta-analysis proposes an overall prevalence of 4.9% [3].
Currently, two direct oral anticoagulants (DOAC), rivaroxaban and dabigatran, have received approval for treating acute venous thromboembolism in children [4]. In the EINSTEIN-Jr phase 3 study, rivaroxaban, a direct factor Xa inhibitor, showed a decrease in thrombotic burden without increasing haemorrhages compared to standard anticoagulants [5,6]. Current experience with the use of DOAC in patients with NS, although limited, suggests these drugs are promising as an alternative for patients with treatment failure or at high risk of adverse effects with classic anticoagulants such as warfarin and heparin. Of note, Xa factor levels might be normal, high, or low in NS. Therefore, the efficacy and the risk of adverse events of treatment with DOAC, such as rivaroxaban, might be difficult to establish [7].
To the best of our knowledge, there are several case reports highlighting the use of DOAC in adults with NS [7], but only one reporting the use of rivaroxaban for the management of venous thromboembolism in children with NS [8]. We believe we are the first to report the use of rivaroxaban in children with CNS. We describe two paediatric patients with CNS who were treated with rivaroxaban. In the absence of antiphospholipid syndrome (Table 1), high risk of bleeding, liver dysfunction or ongoing treatment with CYP3A4 inducers or inhibitors, there were no formal contraindications for the drug [4].
A comprehensive thromboembolic risk assessment was carried out for both patients, revealing only the abnormal findings expected in CNS (Table 1). It is also important to highlight that both patients underwent several CVC replacements, increasing their thrombotic risk. Therapeutic doses were started on both cases according to published recommendations for acute venous thromboembolism in children [5]. No new systemic thrombotic events were diagnosed since starting treatment, and no severe haemorrhages or serious adverse effects were observed. This not only supports the efficacy and safety of the drug as previously described [5,6], but also suggests rivaroxaban might be an option in a subgroup of pediatric patients with CNS.
Robust and larger studies are needed concerning the use of DOAC in children, especially in children with NS and high risk for thrombotic events, such as children with NS and, particularly, CNS. Such studies may contribute to update recommendations for treatment and prevention of thrombotic events among children with NS and CNS.
Learning Points
i. There is a higher prevalence of thrombotic events in CNS compared to other groups of NS.
ii. Rivaroxaban appears to be a safe and effective option for the treatment and prophylaxis of venous thromboembolism in children with CNS.
iii. More research is needed to guide new recommendations about the use of DOAC to reduce the thrombotic burden of CNS without significantly increasing the risk of bleeding.
Acknowledgements
No funding was received to assist with the preparation of this manuscript. Procedures were followed according to the regulations established by the Clinical Research and Ethics Committee and to the Helsinki Declaration of the World Medical Association updated in 2013.
Patient’s Consent
Patient’s consent was obtained prior to publication.
References
- Young G, Lensing A, Monagle P, Christoph M, Kirstin T, et al. (2020) Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein-Jr phase 3 dose-exposure-response evaluation. J Thromb Haemost 18(7): 1672-1685.
- Boyer O, Schaefer F, Haffner D, Dieter H, Detlef B, et al. (2021) Management of congenital nephrotic syndrome: consensus recommendations of the ERKNet-ESPN Working Group. Nat Rev Nephrol 17(4): 277-289.
- Kerlin B, Haworth K, Smoyer W (2014) Venous thromboembolism in paediatric nephrotic syndrome. Pediatr Nephrol 29 (6): 989-997.
- Wang Z, Tang HY, Lin Q, Li XZ (2023) Incident of thromboembolism in children with primary nephrotic syndrome: a systematic review and metanalysis. BMC Nephrol 24: 120.
- Al-Ghafry M, Sharathkumar A (2022) Direct oral anticoagulants in pediatric venous thromboembolism: Review of approved products rivaroxaban and dabigatran. Front Pediatr 10: 1005098.
- Male C, Lensing A, Palumbo J, Riten K, Ildar N, et al. (2019) Rivaroxaban compared with standard anticoagulants for treatment of acute venous thromboembolism in children: a randomized, controlled, phase 3 trial. Lancet Haematol 7(1): e18-e27.
- Sexton D, Freitas D, Little M, Tomas H, Colm M, et al. (2018) Direct-acting oral anticoagulants as prophylaxis against thromboembolism in the nephrotic syndrome. Kidney Int Rep 3(4): 784-793.
- Pelland-Marcotte MC, Tole S, Bouhelier E, Susan L, Jessica H, et al. (2023) Rivaroxaban for management of venous thromboembolism in pediatric nephrotic syndrome; a case report and review of literature. Pediatr Hematol and Oncol 40(7): 688-695.






























