From Seizure to Epilepsy During Hypoxo-Ischemic Encephalopathy: EEG Role
Sahar Chakroun1,2, Sana Ben Jemaa1,2, Nadia Kolsi3, Hela Zouari1,2, Kaouthar Masmoudi1,2 and Nedia Hentati3
1Physiology Department, Sfax University, Tunisia
2Functional explorations Department, Habib Bourguiba Hospital, Sfax, Tunisia
3Neonatology Department, Hesdi Chaker Hospital, Sfax, Tunisia
Submission:April 05, 2023; Published: June 01, 2023
*Corresponding author:Sahar Chakroun, Physiology Department, Sfax University, and Functional explorations Department, Habib Bourguiba Hospital, Sfax, Tunisia
How to cite this article:Sahar C, Sana Ben J, Nadia K, Hela Z, Kaouthar M. From Seizure to Epilepsy During Hypoxo-Ischemic Encephalopathy: EEG Role. Acad J Ped Neonatol 2023; 12(4): 555900. 10.19080/AJPN.2023.12.555900
Abstract
Introduction: Although perinatal care has improved significantly, accurately predicting epilepsy in neonates with hypoxic-ischemic encephalopathy (HIE) remains challenging especially in Resource-limited settings. The primary objective of our study was to investigate the utility of standard conventional electroencephalography (C-EEG) for diagnosing and managing neonatal seizures associated with HIE and its ability to predict epilepsy.
Methods: This retrospective longitudinal study involved 56 full-term newborns who were admitted to the neonatology department for HIE between January 2014 and December 2019. Clinical data was collected, and the C-EEG results were re-analyzed blindly by an experienced electrophysiologist. The evaluation criterion for the study was the presence of epilepsy at 18 months of age.
Results: Out of the 39 video-EEG recordings, non-epileptic phenomena were observed in 10 newborns (17.86%). However, electroclinical seizures were observed in 7 newborns (12.5%) and were associated with electrographic seizures in 6 patients (10.71%). Seizure recurrence was observed in 47.8%(n=22) of newborns, and it was significantly correlated with background activity abnormalities on EEG tracings (p<0.001). We found a significant correlation between epilepsy and EEG immaturity (p=0.031), interhemispheric asynchrony asymmetry (p-0.001), and EEG scores (AUC>0.7). Epilepsy was also significantly correlated with electroclinical seizures (p=0.019) and electrographic seizures (p=0.004). Additionally, periodic lateralized epileptiform discharges (PLEDs) were significantly associated with epilepsy (p=0.019). However, other interictal abnormalities were comparable between the two groups.
Conclusion: The value of continuous EEG in the context of HIE is widely known in the literature. However, this study with markers that are specific to our daily clinical practice, reaffirms the value of standard EEG in resource-limited settings.
Keywords: Hypoxo-Ischemic Encephalopathy; EEG, Prognosis Factors; Epilepsy; Neonatal Seizure; Clonic Seizure; Epileptic Spasm; Myoclonic Seizure; And Tonic Seizures; Non-Motor Seizures; Hyperkplexia; Anti-Epileptic
Abbreviations: HIE: Hypoxo-Ischemic Encephalopathy; EEG: Electroencephalogram; C-EEG: Conventional EEG; PNA: Perinatal Asphyxia; CSE: Clinical Status Epilepticus; WA: Week of Age; BRDS: Brief Rythmic Discharges; PLEDS: Periodic Lateralized Epileptiform Discharges; ROC: Receiver Operating Characteristics ; AUC: Area Under the Cureve
Introduction
HIE occurs in 1 to 1.5 newborns per 1000 live births in developed countries and up to 10 times more in developing countries [1]. Neonatal epileptic seizures are the most frequent clinical manifestation of cerebral dysfunction [2] but determining their epileptic origin during HIE can be challenging due to their variable semiology and sometimes subtle or only electrographic presentation [3]. Therefore, performing an EEG within hours of suspected HIE is essential for both its management and the diagnosis of epileptic seizures [4].
Despite advances in perinatal care, 25% of HIE survivors are at risk of developing neurological disabilities such as cerebral palsy, intellectual disability, and epilepsy [5]. However, establishing an early and certain prognosis of HIE remains difficult in the neonatal period. This prognosis is aided by continuous EEG and new tools of signal analysis [6-9] which may not be available in resourcelimited settings [10]. Thus, they pose a significant clinical challenge for physicians in low- and middle-income countries (LMIC.
In this perspective, our main objective was to evaluate the usefulness of standard conventional EEG (C-EEG) in diagnosing and managing neonatal seizures during hypoxic events and its prognostic value for predicting the development of epilepsy.
Materials and Methods
Patients
This is a retrospective longitudinal study that included 56
newborns who were followed in the neonatology department of
Hedi Chaker University Hospital and the functional explorations
department of Habib Bourguiba University Hospital in Sfax
between January 1, 2014, and December 31, 2019. The study
included newborns with a gestational age of greater than 37
weeks who met the following criteria:
• Diagnosis of encephalopathy based on the presence of
paroxysmal phenomena, neurological distress, and difficulties in
maintaining spontaneous breathing [11]
• A likely hypoxic origin of the encephalopathy based on
the presence of at least one perinatal asphyxia (PNA) criterion
such as obstetric incident, fetal heart rhythm abnormalities,
Apgar score at 5 min lower than 7, need for resuscitation at birth,
a venous pH lower than 7.20 beyond 2 hours of life, meconial fluid,
or signs of respiratory distress [1,5,12-14]
• Availability of clinical follow-up in the neonatal
department
• Initial EEG during the first 15 days of life.
Newborns with infectious, genetic, metabolic, or hematological
diseases were excluded from the study. None of the patients
received hypothermia since it is not available in Tunisia
Methods
Data collection was performed in a double-blind manner by two examiners, with one examiner collecting clinical, biological, and radiological data from medical records, and a second examiner analyzing the EEGs carried out in the functional explorations department.
Clinical Data
We analyzed epidemiological and anthropometric data as well as neurological examinations. The severity of HIE was assessed using the Sarnat score [13,15] All paroxysmal phenomena were recorded, and jerkiness was classified as non-epileptic, while clinical seizures were diagnosed based on the definition of the ILAE [16]. The different types of seizures were classified according to the 2020 ILAE neonatal seizure classification into motor seizures (including automatism, clonic seizure, epileptic spasm, myoclonic seizure, and tonic seizures), non-motor seizures (autonomic seizure and behavioral arrest), sequential seizures, and unclassified seizures [17]. Clinical status epilepticus (CSE) was defined as repeated seizures lasting more than 15 minutes [3] .
We also noted cases of spontaneous resolution, defined as the cessation of paroxysmal manifestations without requiring antiepileptic treatment, as well as the recurrence of paroxysmal phenomena during hospitalization with or without treatment.
Electroencephalographic Data
C-EEG recordings were performed by a digital electroencephalograph of the “Nihon Kohden EEG system”. A minimum duration of 60 minutes recording without artifacts, including a recording of wakefulness, calm sleep, and restless sleep, was required for EEG analysis. The analysis was performed on 20-second pages with longitudinal bipolar montage. The analyzed EEG features were background activity, interictal and ictal abnormalities, as well as the video recordings of paroxysmal phenomena.
Fellow Up
The evaluation criterion for this study was the presence of epilepsy, as defined by the International League Against Epilepsy (ILAE) [18], at 18 months of age.
Statistical Analysis
For statistical analysis, we used version 20 of the Statistical Package for the Social Sciences. We conducted a descriptive study and compared the groups of newborns with and without epilepsy using the appropriate statistical tests such as the chi-square, Fisher’s exact test, t-test, and Mann-Whitney test depending on the type of variables. We set the confidence interval at 95% and considered a p-value of less than 0.05 to be significant. We also performed an analysis of Receiver Operating Characteristics (ROC) curves to compare the predictive value of the scores used. An area under the curve (AUC) greater than 0.7 was considered significant.
Results
We included 56 newborns in our study, with a sex ratio of 2.29. The mean gestational age was 39.54 ± 1.27 weeks.
Seizures Diagnosis
Clinically, seizures were suspected in 46 newborns (82.14%) with a mean onset age of 37.30 ± 40.06 hours. Tremor was reported in 22.64% (n=12) newborns, with 7 cases (58.33%) showing an association with epileptic seizures. EEGs were performed at a mean age sof 5.18 ± 4.19 days. Of the 39 EEG recordings that included a video, paroxysmal phenomena were captured in 7 newborns (43.59%). The non-epileptic nature of the paroxysmal phenomena was confirmed in 10 newborns (17.86%) and was significantly associated with spontaneous resolution (p=0.035). Electroclinical seizures were observed in 7 newborns (12.5 and were associated with electrographic seizures in 6 patients (10.71%). CSE was noted in 6 newborns, with two cases being electroclinical and four cases being electrographic.
Management of Epileptic Seizures
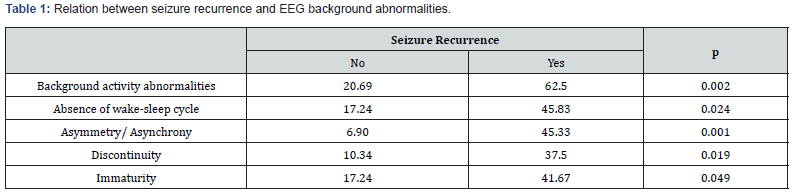
Out of the observed seizures, 30.4% (n=14) had spontaneous resolution, while 69.6% (n=32) required treatment with antiepileptic therapy, either a single dose or two doses of Phenobarbital. antiepileptic treatment was initiated only after EEG seizure detection in five patients, three of whom had electroclinical seizures, and two had CSE. Seizure recurrence was noted in 47.8% (n=22) of cases and was significantly associated with background activity abnormalities on EEG tracings (Table 1).
Of the recurrent seizures, 66.7% (n=12) required treatment with Clonazepam used instead of Phenytoin, which was unavailable. Seven out of twelve newborns who experienced a second seizure recurrence required third-line antiepileptic therapy with Valproate. Drug-resistant seizures were significantly correlated with electrographic seizures (p=0.022), BRDs (p=0.014), and background activity abnormalities (p=0.043). Upon discharge, 14 newborns received anti-epileptic treatment for two months due to uncontrolled seizures.
Epilepsy Prognosis Factors
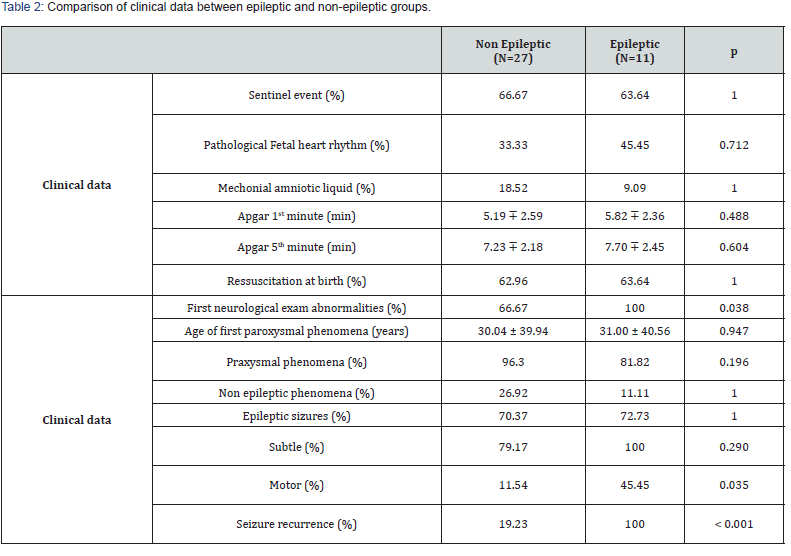
Three patients died before reaching 18 months of age. Among the remaining 53 patients, only 38 newborns were followed up until the age of 18 months. Some patients were transferred to other departments, while others were unable to attend follow-up visits due to the COVID-19 pandemic. We compared the group of newborns with epilepsy (n=11) to those without epilepsy (n=27) in order to determine prognostic factors.
Clinical Factors
No significant relationship was found between the two groups regarding epidemiological and obstetrical data. At admission all newborns who developed epilepsy had neurological abnormalities (p=0.038), while the frequency of neurological abnormalities at discharge was equal between the two groups
Furthermore, Out of the 27 newborns in the non-epileptic group, 24 of them, which accounts for 88.89%, were not prescribed any antiepileptic treatment at the time of discharge (p=0.003). In contrast, all newborns in the epilepsy group (n=11) received antiepileptic treatment since the first seizure, which was maintained until discharge (p<0.001) (Table 2).
Electroencephalographic Factor
In terms of background activity, a significant correlation was observed between epilepsy and EEG immaturity (p=0.031), as well as interhemispheric asynchrony asymmetry (p=0.001) (Table 3).
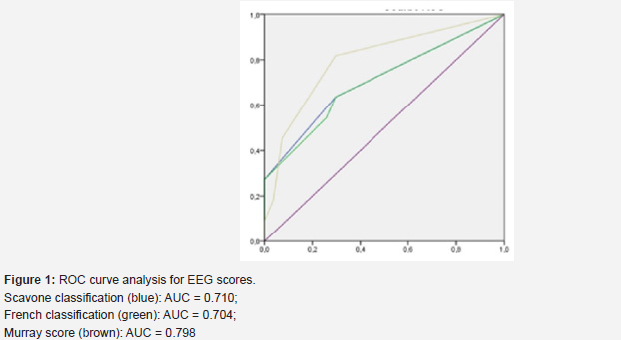
Three studied scores were significantly correlated to epilepsy: Scavone classification (p=0.03), grade 3 of the French classification (p=0.032) and Murray score (p=0.022). These findings were further supported by ROC curve analysis, which yielded a highly significant area under the curve of over 0.7.
Regarding interictal abnormalities, a significant correlation was found between epilepsy and PLEDs (p=0.019). However, there was no significant difference in the incidence of other focal or multifocal interictal abnormalities between the two groups (Figure 1).
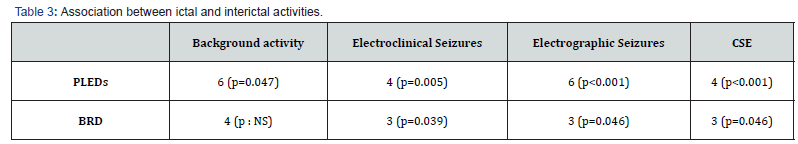
Table 3 displays the association between ictal and interictal activities. The study found that both PLEDs and BRDs were significantly associated with ictal activity. Additionally, newborns with PLEDs associated with electrographic seizures had a significantly higher prevalence of epilepsy (p=0.004).
Epilepsy was also significantly correlated to electro-clinical seizures (p=0.019), electrographic seizures (p=0.004) and CSE (p=0.019) (Table 4).
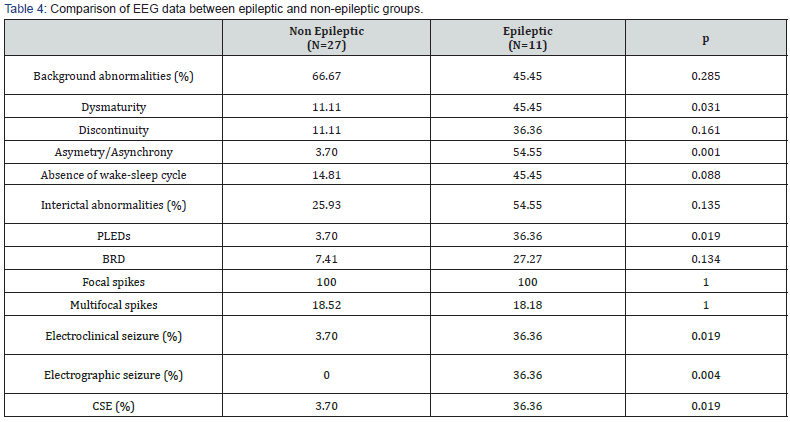
Table Abbreviations: PLEDs: Periodic lateralized epileptiform discharges; BRD: Brief rhythmic discharges; CSE: Clinical status epilepticus.
Discussion
Seizure Diagnosis
Non-Epileptic Phenomena
Various non-epileptic motor phenomena, such as tremors, jerkiness, and less frequently observed physiological sleep myoclonus and hyperkplexia [19], can mimic the clinical signs of an epileptic seizure. The high rate of clinically suspected epileptic seizures (82.14%) compared to the seizures confirmed by video EEG (12.5%) can be attributed in part to the overdiagnosis of epileptic seizures based on clinical signs, as well as the use of standard C-EEG recordings with limited duration, which decreases the likelihood of capturing electroclinical seizures. In fact, the probability of capturing epileptic seizures is higher with prolonged duration of C-EEG recordings [2].
It is crucial to take into consideration the high incidence of non-epileptic phenomena among newborns (17.86% in our study). In the literature, a discrepancy in the diagnosis of neonatal epileptic seizures was also observed depending on the diagnostic criteria used. Studies based on clinical diagnosis reported seizure rates ranging from 65% to 85% [20,21]. However, studies that based their diagnosis on EEG criteria found a lower seizure rate ranging from 30% to 40% [22-26]. Simultaneously recording a video alongside C-EEG enables the analysis of the paroxysmal phenomenon’s semiotics and its potential association with concomitant critical electrical activity, resulting in a 45% increase in the diagnosis of epileptic seizures when adding a video recording to EEG [27].
Electrographic Seizures
During neonatal period, 50 to 80% of the recorded seizures were only electrographic [22,28,29]. In our study, 46.15% of EEGrecorded seizures were only electrographic without any obvious clinical manifestations.
Several hypotheses have been proposed to explain the absence
of clinical manifestations accompanying critical activity [3,17,21]:
• Clinical manifestations may be subtle and difficult to
identify and analyze and may involve non-motor cerebral regions.
• The central nervous system depressant effect of antiepileptic
treatments, particularly phenobarbital, sedative drugs
or therapeutic hypothermia, can lead to a clinical-electrical
dissociation.
• Severe and extensive brain lesions may cause
disconnection of the cortex from its efferent pathways.
CSE
The incidence of CSE in newborns has been poorly studied [32]. The ILAE definition of CSE [17] is partially applicable to newborns, and until 2021, no consensus could be reached regarding the definition of CSE in the neonatal period [29]. This is partly due to the frequent association of neonatal seizures with encephalopathy, making it challenging to assess the baseline neurological status between seizures. Thus, EEG criteria appear necessary for the diagnosis of CSE.
Management of Epileptic Seizures
Initiating and maintaining antiepileptic treatment for electrographic seizures in newborns is still a subject of debate [33,34]. Some studies suggest initiating treatment for electrographic seizures only if they are prolonged or associated with autonomic manifestations [33]. However, other studies strongly support the treatment of electrographic seizures [34,35]. In our study, nine newborns with epileptic seizures had antiepileptic drugs maintained ON long term due to seizures persistence. These seizures seemed to correspond to unprovoked seizures, likely due to structural and genetic abnormalities or severe brain damage following HIE according to Pisani [36].
On the other hand, 8 untreated newborns and 3 newborns treated for only one month did not develop subsequent epilepsy and likely correspond to acute symptomatic seizures self-limited in time, related to an acute cerebral insult such as metabolic or infectious pathology as part of HIE. They disappear in the following days with a low risk of recurrence. Prolonged antiepileptic treatment is not indicated in this case [36].
Prognosis Factors
Clinical Factors
The prognostic value of different types of seizures in HIE remains uncertain. While the presence of epileptic seizures has been linked to a higher risk of developing epilepsy regardless of their clinical semiology[12,37,38], certain types of seizures have been associated with either a favorable or unfavorable outcome. For instance, focal clonic seizures sparing the facial region have been linked to better outcomes [39,40], while myoclonic seizures have been linked to a worse prognosis [40].
In our study, we found a significant association between motor seizures and subsequent epilepsy development (p=0.035). However, no correlation was found between specific seizure types and their progression to epilepsy, likely due to the limited sample size and lack of detailed seizure semiology description. Seizures recurrence, quite common in literature (30 to 50% of cases) [34], was considered as a risk factor for unfavorable outcomes [38,41,42], a finding consistent with our study where all epileptic patients experienced recurrent seizures (p<0.001).
EEG Factors
Background Activity
Many previous studies have not provided detailed information on the specific EEG parameters analyzed and have instead focused on a global description of the background activity [43,44]. Pezzani reported a significant association between a normal initial EEG pattern and a favorable outcome [43], while abnormal background activity [39,45-49], such as the absence of the sleep-wake cycle, interhemispheric asymmetry [22], and dysmaturity [50] has been linked to an unfavorable evolution. The classification of the tracings based on background activity has shown strong prognostic value [51].
Both inactive [43,52] and paroxysmal trace [2,49,53] were associated with an unfavorable evolution.
In our study, we found a significant correlation between an initial inactive EEG and the later development of epilepsy (p=0.048). ROC curve analysis revealed highly significant AUC (>0.7) for the three electrographic scores used, confirming the prognostic value of EEG in predicting later epilepsy.
Interictal Activity
PLEDs have been reported to be associated with electroclinical or electrographic seizures in 40-90% of cases [54]. Furthermore, studies have shown a significant association between PLEDs and epilepsy, particularly if associated with subclinical seizures [54- 56]. These findings were also confirmed in our study.
Due to the high epileptogenic potential of PLEDs, some authors suggest initiating antiepileptic treatment even in the absence of a definite epileptic seizure [57].
The epileptogenic nature of BRDs is still under debate. Although they were not found to be significantly associated with seizures in some studies [58], they may be an early warning signal of possible seizures. Therefore, prolonged C-EEG should be performed to detect electrographic seizures in their presence [17].
Ictal Activity
In our study, the percentage of electroclinical seizures was lower (12.5%) compared to other studies that used prolonged recordings [22,26], but we still found a significant association between epilepsy and both electroclinical and electrographic seizures. This is consistent with findings from other studies [22,43,59,60]. However, there are also studies that did not find a significant association between seizures and epilepsy [12,20,25,45,46,53].
It’s important to note that the presence of a CSE is highly predictive of an unfavorable outcome, including the development of epilepsy [32,41]. In our study, we also found that all newborns with CSE had developed epilepsy, which further supports this association (p=0.019).
Limits
As a retrospective study, there were limitations in the availability of clinical and paraclinical data in the medical records. Additionally, some patients were lost to follow-up due to the impact of the COVID-19 pandemic.
Conclusion
Undoubtedly, EEG has been extensively investigated for its diagnostic and prognostic value in HIE in the literature. However, this study is the first in our region to examine the contribution of C-EEG in HIE. Our findings provide more accurate markers for daily clinical practice, considering the diagnostic and therapeutic resources available in resource-limited settings. Our study confirms the usefulness of C-EEG with both visual analysis and scorebased analysis and highlights the importance of incorporating video into C-EEG monitoring. Furthermore, C-EEG can guide the decision to perform prolonged video recordings in specific cases. Advancements in technology and resource availability can significantly impact the management and prognosis of HIE patients. Therefore, it is crucial to continue improving existing settings with simple interventions such as ensuring easier access to EEG and incorporating video into C-EEG monitoring. These measures can have a considerable impact on the management of HIE and subsequent prognosis for newborns in Tunisia.
References
- Hankins G (2003) Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol 102(3): 628‑6
- Lai YH, Ho CS, Chiu NC, Tseng CF, Huang YL (2013) Prognostic Factors of Developmental Outcome in Neonatal Seizures in Term Infants. Pediatr Neonatol 54(3): 166‑1
- Kaminska A, Mourdie J, Barnerias C, Bahi-Buisson N, Plouin P, et al. (2007) Conduite à tenir en cas de « convulsions » néonatales. Arch Pédiatrie 14(9): 1137‑11
- Walsh BH, Murray DM, Boylan GB (2011) The use of conventional EEG for the assessment of hypoxic ischaemic encephalopathy in the newborn: A review. Clin Neurophysiol 122(7): 1284‑12
- Volpe JJ (2008) Neurology of the newborn (5th edn), Saunders/Elsevier, Philadelphia, USA, p. 1094.
- Kota S, Massaro AN, Chang T, Al-Shargabi T, Cristante C, et al. (2020) Prognostic Value of Continuous Electroencephalogram Delta Power in Neonates With Hypoxic-Ischemic Encephalopathy. J Child Neurol 35(8): 517‑5
- Lacan L (2021) Analyse quantitative et automatisée des EEG néonataux post-anoxiques: développement d’un outil clinique d’aide au diagnostic précoce de l’encéphalopathie anoxo-ischémique néonatale.
- O’Toole JM, Mathieson SR, Raurale SA, Magarelli F, Marnane WP, et al. (2023) Neonatal EEG graded for severity of background abnormalities in hypoxic-ischaemic encephalopathy. Sci Data 10(1): 129.
- Chock VY, Rao A, Van Meurs KP (2023) Optimal neuromonitoring techniques in neonates with hypoxic ischemic encephalopathy. Front Pediatr 11: 1138062.
- Vegda H, Krishnan V, Variane G, Bagayi V, Ivain P, et al. (2022) Neonatal Seizures—Perspective in Low-and Middle-Income Countries. Indian J Pediatr 89(3): 245‑2
- Shevell MI (2004) The “Bermuda triangle” of neonatal neurology: cerebral palsy, neonatal encephalopathy, and intrapartum asphyxia. Semin Pediatr Neurol 11(1): 24‑
- Pisani F, Orsini M, Braibanti S, Copioli C, Sisti L, et al. (2009) Development of epilepsy in newborns with moderate hypoxic-ischemic encephalopathy and neonatal seizures. Brain Dev 31(1): 64‑6
- Sarnat HB, Sarnat MS (1976) Neonatal Encephalopathy Following Fetal Distress: A Clinical and Electroencephalographic Study. Arch Neurol 33(10): 696-704.
- Amiel-Tison C, Ellison P (1986) Birth asphyxia in the fullterm newborn: early assessment and outcome. Dev Med Child Neurol 28(5): 671-682.
- AMERICAN ACADEMY OF PEDIATRICS COMMITTEE ON FETUS AND NEWBORN, AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS COMMITTEE ON OBSTETRIC PRACTICE (2015) The Apgar Score. Pediatrics 136(4): 819‑8
- Fisher RS, Boas W van E, Blume W, Elger C, Genton P, et al. (2005) Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46(4): 470‑47
- Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, et al. (2021) The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia 62(3): 615-628.
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, et al. (2014) ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 55(4): 475‑4
- Marret S, Chadie A, Rondeau S, Lebas A (2012) Mouvements anormaux paroxystiques du nouveau-né à terme. Arch Pédiatrie 19(6): H205‑H20
- Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL (1981) Hypoxic-ischemic encephalopathy in term neonates: Perinatal factors and outcome. J Pediatr 98(1): 112‑11
- Al-Macki N, Miller SP, Hall N, Shevell M (2009) The Spectrum of Abnormal Neurologic Outcomes Subsequent to Term Intrapartum Asphyxia. Pediatr Neurol 41(6): 399‑
- Murray DM, Boylan GB, Ryan CA, Connolly S (2009) Early EEG Findings in Hypoxic-Ischemic Encephalopathy Predict Outcomes at 2 Years. Pediatrics 124(3): e459‑e4
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, et al. (2009) Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr 155(3): 318‑3
- Miller SP, Latal B, Clark H, Barnwell A, Glidden D, et al. (2004) Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol 190(1): 93-99.
- Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM (1999) Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol 21(5): 788-793.
- d’Allest A, André M, Radvanyi-Bouvet M (1996) Apport de l’électroencéphalogramme pour le diagnostic et le pronostic de l’asphyxie périnatale du nouveau-né à terme. Arch Pédiatrie 3: S254‑S25
- Pisani F, Spagnoli C (2019) Diagnosis and Management of Acute Seizures in Neonates. In: Neurology [Internet]. Elsevier p. 111‑1
- Scher MS (2006) Neonatal seizure classification: A fetal perspective concerning childhood epilepsy. Epilepsy Res 70(Suppl 1): 41‑
- McBride MC, Laroia N, Guillet R (2000) Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 55(4): 506‑5
- Mizrahi EM, Kellaway P (1987) Characterization and classification of neonatal seizures. Neurology 37(12): 1837‑
- de Corrêa NC, Bom JM da S, Scherer MR, Nunes ML (2022) Clinical profile of a cohort of neonates with seizures: Association between semiology, etiology, and electroencephalographic findings. Pediatr Neonatol 63(6): 582‑58
- Pisani F, Cerminara C, Fusco C, Sisti L (2007) Neonatal status epilepticus vs recurrent neonatal seizures: Clinical findings and outcome. Neurology 69(23): 2177‑21
- Silas R, Sehgal A, Walker AM, Wong FY (2012) Cerebral oxygenation during subclinical seizures in neonatal hypoxic-ischaemic encephalopathy. Eur J Paediatr Neurol 16(3): 304‑30
- Glass HC (2014) Neonatal Seizures. Clin Perinatol. Mars 41(1): 177‑1
- World Health Organization (2011) Guidelines on neonatal seizures [Internet]. World Health Organization, Geneva, p. 95.
- Pisani F, Spagnoli C, Falsaperla R, Nagarajan L, Ramantani G (2021) Seizures in the neonate: A review of etiologies and outcomes. Seizure 85: 48‑
- Nunes ML, Martins MP, Barea BM, Wainberg RC, Costa JC da (2008) Neurological outcome of newborns with neonatal seizures: a cohort study in a tertiary university hospital. Arq Neuropsiquiatr 66(2a): 168‑1
- Altunbaşak Ş, Incecik F, Hergüner Ö, Refik Burgut H (2007) Prognosis of Patients With Seizures Occurring in the First 2 Years. J Child Neurol 22(3): 307‑3
- Garfinkle J, Shevell MI (2011) Prognostic factors and development of a scoring system for outcome of neonatal seizures in term infants. Eur J Paediatr Neurol 15(3): 222‑22
- Ronen GM, Buckley D, Penney S, Streiner DL (2007) Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 69(19): 1816-1822.
- Kang SK, Kadam SD (2015) Neonatal Seizures: Impact on Neurodevelopmental Outcomes. Front Pediatr 3: 101.
- Dixon G, Badawi N, Kurinczuk JJ, Keogh JM, Silburn SR, et al. (2002) Early Developmental Outcomes After Newborn Encephalopathy. Pediatrics 109(1): 26‑
- Pezzani C, Radvanyi-Bouvet MF, Relier J, Monod N (1986) Neonatal Electroencephalography During the First Twenty-Four Hours of Life in Full-Term Newborn Infants. Neuropediatrics 17(01): 11‑1
- Biagioni E, Bartalena L, Boldrini A, Pieri R, Cioni G (1999) Constantly discontinuous EEG patterns in full-term neonates with hypoxic-ischaemic encephalopathy. Clin Neurophysiol 110(9): 1510‑151
- Mercuri E, Rutherford M, Cowan F, Pennock J, Counsell S, et al. (1999) Early Prognostic Indicators of Outcome in Infants with Neonatal Cerebral Infarction: A Clinical, Electroencephalogram, and Magnetic Resonance Imaging Study. Pediatrics 103(1): 39‑
- Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, et al. (2016) Seizure burden and neurodevelopmental outcome in neonates with hypoxic–ischemic encephalopathy. Dev Med Child Neurol 58(12): 1242‑124
- Laroia N, Guillet R, Burchfiel J, McBride MC (1998) EEG Background as Predictor of Electrographic Seizures in High-Risk Neonates. Epilepsia 39(5): 545‑5
- Ramantani G (2013) Neonatal epilepsy and underlying aetiology: to what extent do seizures and EEG abnormalities influence outcome? Epileptic Disord 15(4): 11.
- Khan RL, Lahorgue Nunes M, Garcias da Silva LF, da Costa JC (2008) Predictive Value of Sequential Electroencephalogram (EEG) in Neonates With Seizures and Its Relation to Neurological Outcome. J Child Neurol 23(2): 144‑1
- Rener-Primec Z, Neubauer D, Osredkar D (2022) Dysmature patterns of newborn EEG recordings: Biological markers of transitory brain dysfunction or brain injury. Eur J Paediatr Neurol 38: 20‑2
- Tekgul H (2006) The Current Etiologic Profile and Neurodevelopmental Outcome of Seizures in Term Newborn Infants. Pediatrics 117(4): 1270‑12
- Scavone C, Radvanyibouvet M, Morelkahn F, Dreyfusbrisac C (1985) Coma apres souffrance foetale aigue chez le nouveau-ne a terme: Evolution electro-clinique. Rev DaposElectroencéphalographie Neurophysiol Clin 15(3): 279‑2
- Menache CC, Bourgeois BFD, Volpe JJ (2002) Prognostic value of neonatal discontinuous EEG. Pediatr Neurol 27(2): 93‑
- Tao JX, Qin X, Wang Q 2020() Ictal-interictal continuum: a review of recent advancements. Acta Epileptol 2(1): 13.
- Punia V, Garcia CG, Hantus S (2015) Incidence of recurrent seizures following hospital discharge in patients with LPDs (PLEDs) and nonconvulsive seizures recorded on continuous EEG in the critical care setting. Epilepsy Behav 49: 250‑25
- McCutchen CB, Coen R, Iragui VJ (1985) Periodic lateralized epileptiform discharges in asphyxiated neonates. Electroencephalogr Clin Neurophysiol 61(4): 210‑21
- Punia V, Vakani R, Burgess R, Hantus S (2018) Electrographic and Clinical Natural History of Lateralized Periodic Discharges: J Clin Neurophysiol 35(1): 71‑7
- Yoo JY, Marcuse LV, Fields MC, Rosengard JL, Traversa MV, Gaspard N, et al. (2017) Brief Potentially Ictal Rhythmic Discharges [B(I)RDs] in Noncritically Ill Adults. J Clin Neurophysiol 34(3): 222‑22
- Connell J, Oozeer R, de Vries L, Dubowitz LM, Dubowitz V (1989) Continuous EEG monitoring of neonatal seizures: diagnostic and prognostic considerations. Arch Dis Child 64(4 Spec No): 452‑45
- Lombroso CT (1996) Neonatal Seizures: Historic Note and Present Controversies. Epilepsia 37(s3): 5‑






























