An Infant with Toxoplasmosis Retinochoroiditis: A Case Report from the Philippines
James E. Eullaran1, Ma. Delta S. Aguilar1,2 and Genelynne J. Beley1,2*
1Southern Philippines Medical Center, Department of Pediatrics, Davao City, Philippines
2Davao Medical School Foundation, Inc, College of Medicine, Department of Pediatrics, Davao City, Philippines
Submission: May 09, 2023; Published: May 17, 2023
*Corresponding author: Genelynne J. Beley, Southern Philippines Medical Center, Department of Pediatrics, Davao City, Philippines,Email:gen_juruena@yahoo.com
How to cite this article:James E. E, Ma. Delta S. A, Genelynne J. B. An Infant with Toxoplasmosis Retinochoroiditis: A Case Report from the Philippines. Acad Acad J Ped Neonatol 2023; 12(4): 555899. 10.19080/AJPN.2023.12.555899
Abstract
Toxoplasmosis is a major and preventable cause of severe visual handicap and blindness among young people. More specifically, Toxoplasmosis retinochoroiditis (TRC) caused by Toxoplasma gondii is a rare but serious manifestation of toxoplasma infection. There are only two probable means of acquiring the disease, through the congenital route and acquired. In the Philippines, there are only a few reported cases and studies about TRC in the human population. With this, there is also a paucity of available therapy for this condition. Diagnostic tools for TRC are not readily available in most areas in the Philippines. This report is a classic Toxoplasma infection in a 9-month-old patient presenting with subtle eye symptoms. The use of serologic markers supported the initial impression.
Keywords: Toxoplasmosis, Toxoplasmosis Retinochoroiditis, parasite, wandering eyes, strabismus
Abbreviations: TRC: Toxoplasmosis retinochoroiditis; SPMC: Southern Philippines Medical Center; TMP-SMX: Trimethoprim-Sulfamethoxazole; ELISA: Enzyme-linked immunosorbent assay
Introduction
In pediatrics, strabismus is a common condition. Males and females may have strabismus, and may have genetic influences [1]. An infant’s ability to control their eye movement should be fully developed by the age of four months. A child with strabismus, will have an eye that is out of alignment, which causes a discrepancy in the visual impulses reaching the brain. Loss of depth perception will occur because the brain will ignore the image of the misaligned eye and concentrate on the image of the straight eye [2]. It is crucial to detect strabismus in children since it can lead to visual and emotional problems. Children who are at high risk for eye problems should be referred to an ophthalmologist for a screening exam. It is crucial to identify children who have obvious strabismus since, if untreated, they have a higher risk of amblyopia. The development of strabismus is multifactorial with both genetic and environmental influences. There are congenital and acquired forms of strabismus, and it’s critical to distinguish between the two because some acquired forms of strabismus pose a risk to one’s life or vision. Birth history, genetics, neuromuscular disorders, malignancies, structural eye problems, trauma, infections, systemic ailments with eye-threatening symptoms, and iatrogenic causes are the prevalent risk factors associated with strabismus among pediatric age groups [2].
Case Description
This is a case of a 10-months old, female infant from Surigao, Philippines, who was brought for evaluation due to occasional wandering of the left eye or squinting. The patient has been apparently well until at four months of life, when occasional wandering of the left eye was noted. No consultation was done for this. However, the wandering of the eye persisted until the patient was 9 months old. There was no history of trauma, leukocoria, eye redness, fever, or seizures. They sought consult at an eye center in their locality. Two ophthalmologists were able to examine her. A “white matter” on the patient’s left eye was seen, hence B scan was requested.
B scan done showed a macular scar versus beginning ocular mass. Follow-up consults was advised.
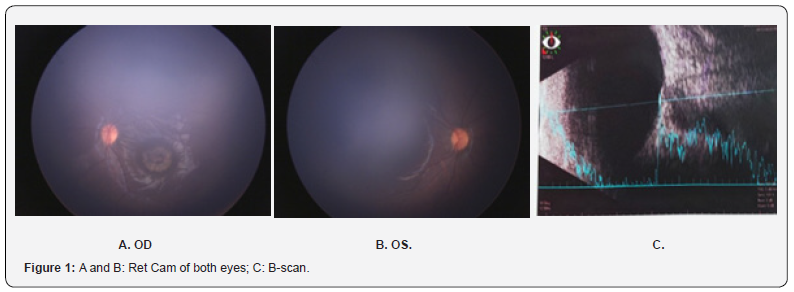
Two months prior to consultation, a Ret Cam was done at Southern Philippines Medical Center (SPMC) where a characteristic calcification was noted (Figure 1). The Ophthalmology service strongly considered a parasitic cause of the eye condition linked to a certain zoonotic parasite. Further history revealed presence of adopted eight cats, some are stray with six dogs, of which two are Belgian Malinois, and six are native-origin dogs. With this, a serum IgM and IgG titer were done with subsequent referral to the pediatric infectious service.
Prenatal and birth history were unremarkable with normal Expanded Newborn Screening and Hearing Tests. Patient was previously diagnosed with COVID-19 mild along with all of the seven family members. The family underwent quarantine protocols. Feeding and immunization status were according to the recommendation of the Philippine Pediatric Society adequate for her age. Developmental milestones are at par with age and family history has hypertension on both maternal and paternal side. Pertinent social history is the family’s love for pets. They have eight cats which roam inside the house and around the village. Moreover, the patient’s grandfather regularly feeds their own cats as well as stray cats. They also have six dogs. All the dogs are vaccinated with anti-rabies and dewormed. However, the pet cats were not.
On physical examination, patient was examined at the outpatient department with stable vital signs: blood pressure of 90/60 mm Hg, heart rate of 102 beats per minutes, respiratory rate of 22 cycles per minute, temperature of 36.8 degrees Celsius. Patient’s weight is 8 kilograms and length of 71 centimeters revealing a normal anthropometric measurement.
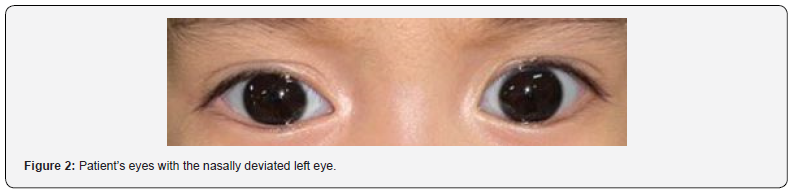
Pertinent findings of the eyes revealed presence of white sclera and pinkish palpebral conjunctiva. Pupils are 4mm in size constricting to 2mm, equally round and reactive to light and accommodations. There is a noticeable inward deviation of the left eye (Figure 2). Detailed ophthalmologic examination by the Ophthalmology Department revealed presence of whitish flat lesion with smooth surface and irregular borders at the area of the macula on the left eye (Table 1). The rest of the systems are unremarkable.
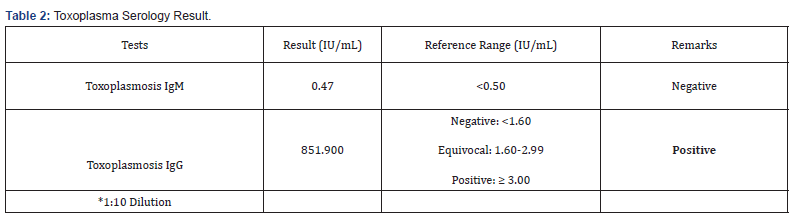
Patient’s work-ups were done as ambulatory. Toxoplasmosis titer for IgG revealed positive confirming the infection of Toxoplasmosis retinochoroiditis (Table 2).
Patient was subsequently managed as a case of Toxoplasmosis retinochoroiditis and was referred to Pediatric Infectious Disease Service for co-management. Cranial CT scan was requested to further rule out extra-organ seeding of the disease. Treatment plans include starting her on Pyrimethamine 2mkD loading dose and 1mkD maintenance dose for 2 months, Trimethoprim- Sulfamethoxazole (TMP-SMX) 12mkD twice a day for 2 months, folic Acid 1mkD once a day for 2 months, and Complete Blood Count monitoring every 2 weeks once the patient is on treatment. The unavailability of Pyrimethamine in the Philippines is a challenge, hence intravenous TMP-SMX administration in lieu of Pyrimethamine is the next option.
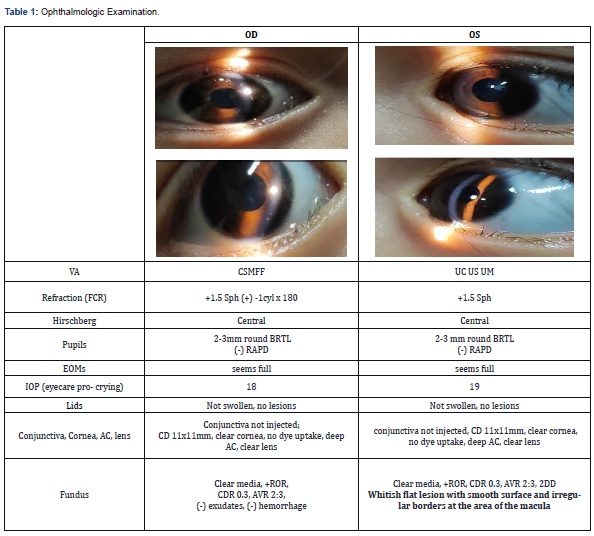
OD: right eye OS: left eye
Discussion
Toxoplasmosis is an infection of the retina caused by the protozoan Toxoplasma gondii that affects both immune-suppressed individuals and healthy individuals globally. It is lethal for those with weakened immune systems. Children with immunologically sound immune systems may have no symptoms from an acute acquired infection, which can also result in lymphadenopathy and may affect other organs [3]. T. gondii, a member of the phylum Apicomplexa, has a polar apical complex that mediates attachment to the host cell membrane. Once acquired, the latent encysted organisms will persist in the host throughout life.
Bone marrow transplant recipients may develop systemic illness as a result of immune-compromised patients regularly experiencing CNS-related signs and symptoms. Congenitally acquired toxoplasmosis can cause chorioretinitis and abnormalities in the CNS during pregnancy or later in life if it is left untreated [3].
The parasite is spread by eating uncooked meat that contains T. gondii cysts or feces-derived oocyst-containing water.7 In countries with a range of cultural, socioeconomic, and ethnic origins, the illness has been seen to spread through both water and food. It is possible for the parasite to spread vertically, most frequently in situations of primary sickness in pregnant women. Despite being rare, transplanted organs have the potential to spread the illness. The most likely cause of the sickness in this case is swallowing a medium containing oocyst from their pet cats’ litters [3]. The patient’s family owns 10 cats at home and this is likely the source of the patient’s infection.
T. gondii is a single-cell, obligate, intracellular protozoan parasite. The cats are the definitive host but humans and several of mammals, birds, and reptiles, may also serve as intermediate hosts. T. gondii has three forms: a. the oocyst (soil form), b. the tachyzoite (active infectious form), and c. the tissue cyst (latent form) [4]. This coccidian protozoan multiplies in living cells. The tachyzoites are 2-4 x 4-7 um and are oval and crescent-shaped. Skeletal muscles, central nervous system and cardiac muscles are the areas where tissue cysts, 10-100 um in diameter are found [5]. These cysts will remain in those tissues [3]. The T. gondii life cycle is divided into two parts: an asexual phase, which takes place in nucleated cells; and a sexual phase through schizogonic and gametogenic cycles within the distal ileal epithelium of the cat intestine. Fertilized gametes are created during sexual reproduction in the feline’s small intestine and are then ejected as two-sporocyst oocysts, which may survive in the environment for up to 18 months with the right humidity and temperature. Each sporocyst matures into four sporozoites. For about two weeks, the cat excretes 105 to 107 oocysts daily. Oocysts turn infectious after sporulating 1–5 days after release. When swallowed oocysts or tissue cysts rupture, they enter the intestinal lining cells and release sporozoites or bradyzoites, which later transform into tachyzoites, the parasite’s form that reproduces quickly. The host’s leukocytes may contain these tachyzoites or they may be freely circulating through the circulation. Once the intestinal mucosa has been breached, tachyzoites move through the patient’s blood and lymphatic system to distant organs like the brain, liver, spleen, lymph nodes, heart, skeletal muscles, placenta, and eyes. Another hypothesis is that the parasite enters the brain and eye after being directed by dendritic cells and macrophages over the blood-brain barrier [3].
Epidemiology
Worldwide, over 6 billion people have been infected with T. gondii. Seroprevalence, measured by IgG against T. gondii, varies in different countries, 6.7% in Korea [6], 12.3% in China [7], 23.9% in Nigeria [8], 46% in Tanzania [9] and 47% in France (rural area), and it can be as high as 98% in some regions [10]. Reports with low seroprevalence are from Southeast Asia, North America and Northern Europe with 10-30%. Prevalence between 30 and 50% have been reported for Central and Southern Europe, whereas high seroprevalences are observed in Latin America and in tropical African countries. The different seropositivity rates largely depends on the prevalence of T. gondii cysts and oocysts in the environment [11]. This infection is more common in countries with humid temperatures such as Asia, the Caribbean region and in Central America [12].
It commonly occurs in healthy children or young adults in 75% of cases, often during puberty. Toxoplasmosis gondii has an affinity for the nervous tissue, particularly involving the retinal ganglion cells in the eye [13].
One study was conducted in Cebu. out of 924 participants, 244 (26.4%) were found to be seropositive from 21 different municipalities and cities in Cebu. Seropositivity was found to be greater among women (27%) than men (25.5%) [11]. For toxoplasmosis retinochoroiditis alone, the seropositivity is between 20% to 70% in the United States while the incidence of Toxoplasma retinochoroiditis is 0.6% [14]. Several studies from around the world have shown that ocular toxoplasmosis is the most common form of posterior uveitis. In certain groups, ocular toxoplasmosis is the primary cause of uveitis. Ocular toxoplasmosis is likely underdiagnosed in many countries with endemic T. gondii infection [15]. It has long been thought that postnatal infections seldom cause symptoms and that prenatal infections are the main cause of ocular toxoplasmosis. This paradigm was challenged by research from Brazil that revealed postnatal infection and toxoplasmosis ocular symptoms were more common than congenital infection. This finding was confirmed by other groups and likely applies to most populations exposed to T. gondii [15].
Clinical Features
When ocular toxoplasmosis manifests with the traditional ophthalmological symptoms, a clinical examination is employed to determine the diagnosis rather than a laboratory test to confirm parasite infection. A history of systemic exposure to the parasite is indicated by toxoplasmosis seropositivity, however this information is inadequate to make the diagnosis. Although retinal lesions in the retina’s periphery frequently result to secondary severe vitreous inflammation and vision loss, macular lesions may also cause secondary visual impairment. Optic nerve involvement can cause substantial visual field anomalies and color vision loss, though less frequently. Active lesions’ primary symptom, which is vision blurring, is connected to symptomatic vitreous inflammation. The size and location of retinochoroidal scars are negatively connected with scotomas during the parasite’s latent stage [14].
A nidus of fluffy white, focal necrotizing retinitis or retinochoroiditis next to a chorioretinal scar with a range of pigmentations is the classic visual presentation of toxoplasmosis.
The traditional “headlight in the fog” indication is frequently caused by a strong vitritis that hides the active lesion. The posterior segment involvement may be hidden by the anterior uveitis, which can range in intensity from a mild response to a severe inflammation. Other common clinical signs of ocular toxoplasmosis include satellite lesion, retinochoroidal scar, focal or widespread vasculitis, and inflammatory ocular hypertension syndrome [14]. Multifocal retinochoroiditis, low-grade or absent vitreal infiltration, an active lesion larger than two disk diameters without an accompanying retinochoroidal scar, absence of a retinochoroidal scar, bilaterality, involvement of the optic disk, choroiditis without retinitis, hemorrhagic vasculitis, serous retinal detachment, and retinal neovascularization are examples of abnormal findings [14]. The patient in this case presented with a macular scar versus beginning intraocular mass with calcifications highly characteristic of toxoplasmosis.
In cases of congenital toxoplasmosis, the mother may have been asymptomatic or have had mild mononucleosis-type symptoms. In contrast, the newborn may exhibit with prematurity, intrauterine growth restriction, jaundice, hepatosplenomegaly, myocarditis, pneumonitis, various rashes and neurologic findings that include chorioretinitis, hydrocephalus, intracranial calcifications, microcephaly, and seizures. Some of the neurological symptoms may only become apparent months to years later. The classic triad of neurological findings consists of chorioretinitis, hydrocephalus, and intracranial calcifications [12].
Toxoplasmosis Retinochoroiditis should be differentiated from other conditions such as retinoblastoma which presents commonly with leukocoria and with a biopsy finding of Flexner- Wintersteiner rosettes4 characteristic of the malignancy. Intake or exposure to lead and other toxins should be ascertained. It can cause neurologic manifestations and could eventually lead to ocular symptoms such as strabismus [16]. Guillain-Barre, Myasthenia Gravis, and Multiple Sclerosis are neuromuscular autoimmune disorders which manifest also with strabismus [17]. However, extraocular manifestations are more prominent in these cases. Presence of specific antibodies supports its diagnosis.
Diagnosis
Basing in the clinical presentation, the diagnosis of ocular toxoplasmosis is frequently obvious. When a funduscopic examination cannot definitively determine the clinical diagnosis, serological testing, such as serum anti-Toxoplasma titers of IgM and IgG, may be required to support the diagnosis. T. gondii antibody titers in ocular fluids or polymerase chain reaction (PCR) of aqueous and vitreous samples are other newer tools with high sensitivity and specificity to confirm the diagnosis [3,14].
The majority of diagnostic laboratories can only use commercial ELISA (enzyme-linked immunosorbent assay) or immunofluorescent antibody kits to measure IgG and IgM antibody levels. Because ELISA allows for automation and the simultaneous testing of numerous samples, it is preferable to immunofluorescent antibody testing because the results are objective. The Sabin- Feldman dye test, the classic gold standard serology test, uses live T. gondii tachyzoites to detect IgG antibodies.
Serum IgM and IgG antibodies to T. gondii develop within 1-2 weeks after infection. IgG serology may be the first test performed on patients suspected of having acute toxoplasmosis; if IgG results are positive, IgM antibody levels may also be assessed. In an immunocompetent patient, nonreactive IgG rules out the toxoplasmosis diagnosis. IgM levels increase during the first week and decrease after 6 to 9 months. A recent infection should not be inferred just from elevated antibody levels, and an inactive illness should not be inferred from low blood IgG levels. Levels of serological tests should be redone in 15–21 days if the lab results are clear [15].
A latent infection with a history of initial exposure may exist in asymptomatic individuals with IgG reactivity alone. This serological pattern determines the likelihood of illness recurrence in immunosuppressed individuals, including those with HIV infection and transplant recipients [8]. In patients with reactivation disease, IgM and IgG response may not be seen. Additional tests to rule out Toxoplasma infection should be carried out in immunocompromised patients with seronegativity but strong clinical evidence. These consist of T. gondii PCR of the vitreous and aqueous humor or IgG antibody testing [15].
The patient in this case has a positive Toxoplasma IgG titer and normal borderline high Toxoplasma IgM titer. Recently, serologic tests served as the primary means of diagnosis. We can conclude that this patient has a toxoplasma infection since IgG specific antibodies achieve a peak concentration 3 to 5 months after infection and remain positive indefinitely. This coincides with the patient’s symptom presentation which started 5 months prior to consultation. With the negative IgM serologic titer, this is not a recent, chronic latent infection, or a false-positive reaction.
The clinical features of a group of patients with ocular toxoplasmosis were compared in research by Rodanes et al. based on the patients’ IgM status. It should be highlighted that the group that tested positive for IgM at presentation had bigger lesions, a higher incidence of macular involvement, and higher levels of intraocular inflammation. In contrast to this study, the patient had a normal or borderline high IgM titer and no additional inflammatory signs [18].
Assessment of the risk of transplacental transfer also uses serology. In nations where toxoplasmosis is an endemic disease, IgG serology is regularly conducted on women who are contemplating pregnancy. Immunocompetent women who have elevated IgG levels before to conception have a reduced likelihood of transplacental transfer. It is suggested that those with undetectable IgG levels stay away from eating raw meat or cat excrement. Acute infection in the last six months is excluded by negative IgM serology; if it is positive, it may linger for up to two years following T. gondii exposure [18].
Biopsy is not frequently done but in atypical cases, it helps in the diagnosis. A necrotizing retinitis occurs with vasculitis and destruction of the retina is commonly appreciated [4].
Treatment and Management
Except in cases where it is proven that the infection occurred during pregnancy, there is ocular involvement, or the symptoms are severe or persistent, the majority of cases of acquired acute toxoplasmosis in immunocompetent hosts do not require specific therapy. Moreover, treatment of acute toxoplasmosis in immunocompromised patients is always recommended. Given this, treating the patient’s condition is only prudent. For children with active toxoplasmosis chorioretinitis, treatment should be initiated right away since it is a medical emergency. When the disease is still in its active stage, they are administered along with pyrimethamine, sulfadiazine, and leucovorin for about a week after the lesion has assumed a quiescent appearance, such as sharp borders, pigmentation at the lesion’s margins, and resolution of associated inflammatory cells in the vitreous, which typically takes place in 2-4 weeks when treatment is initiated promptly. The edges of the retinal lesions get sharper after 7–10 days, and visual acuity becomes normal. When the macula, optic nerve head, or retina are affected, corticosteroids are given together with antibacterial therapies [19]. This should not be given alone and must be started after the initiation of pyrimethamine and sulfadiazine for 2 days. As for this patient, pyrimethamine, TMP-SMX, and Folic acid are proposed to be started. With difficulty in the procurement of pyrimethamine, intravenous route of administration of TMP-SMX in lieu of pyrimethamine is an alternative. TMP-SMX in intravenous form has been reported to be equivalent to pyrimethamine/ sulfadiazine.
Performing a vitrectomy and removing the lens to improve vision is extremely uncommon. In addition to oral anti-toxoplasma medications, intravitreal injection of an antibody to vascular endothelial growth factor has been effective for those with active choroidal neovascular membranes [3].
Prognosis
Within a few years of the initial infection, the likelihood of toxoplasmosis retinochoroiditis reactivation from pre-existing chorioretinal scars increases, with a relatively rapid decline in the incidence of reactivations over time. According to one study, TRC recurrence occurs at a rate of 50% over three years and 80% over five years, with an average lifetime recurrence of 2.7 episodes [3,14]. Other anticipated outcomes include vision impairment of less than 20/40. Additionally, there was a significant decline in verbal and geometric design aspects of the intelligence quotient [20].
Conclusion
The majority of cases of toxoplasmosis are avoidable. One of its consequences, toxoplasmosis retinochoroiditis, is extremely uncommon and challenging to treat. Therefore, it is necessary to follow hygienic procedures at first. Litter boxes and areas frequented by cats, particularly sand or soil in gardens or playgrounds, present a serious risk and have to be avoided. It is advised to regularly clean fruits and vegetables and to wash your hands after coming into touch with soil or cat litter boxes. Food should be handled properly since toxoplasma cysts are destroyed at 60 degrees C for 15 minutes or by -20 degrees C for at least 24 hours. Oocyst-tainted water is now a widely recognized way to contract acquired toxoplasmosis in places with insufficient water treatment. However, an interprofessional team composed of an ophthalmologist, neurologist, and infectious disease specialist is best suited to manage toxoplasmosis retinochoroiditis if it has already manifested. Immunosuppressed patients typically require long-term care and monitoring. Preventive measures including staying away from cat-friendly environments, must be explained to patients and primary caregivers. Patients with TRC have a cautious outlook.
Declaration of Competing of Interest
Declaration of Competing of InterestThe authors declare no conflicts of interest regarding this manuscript..
Funding
FundingThis manuscript received no external funding.
Acknowledgement
The authors would like to thank the residents and consultants of the Department of Pediatrics, Southern Philippines Medical Center.
References
- Wright KW, Spiegel PH, Thompson LS (2006) Handbook of Pediatric Strabismus and Amblyopia. (2nd edn), Springer Science and Business Media Inc., New York, USA.
- Weinstock VM, Weinstock DJ, Kraft SP (1998) Screening for childhood strabismus by primary care physicians. Can Fam Physician 44: 337-343.
- Md RKM, Geme SJ (2019) Nelson Textbook of Pediatrics. (Volume 2 Set), NelsonPediatrics (21st edn) (E-book), Elsevier.
- David CG, Russell WR, Alan P, Jessica S, Marissa L, et al. (2023) Toxoplasmosis. American Academy of Ophthalmology.
- Md EKC, FRCPath KVMM (2021) Robbins and Cotran Review of Pathology (Robbins Pathology) (5th)
(E-book), Elsevier. - Shin DW, Cha DY, Hua QJ, Cha GH, Lee YH (2009) Seroprevalence of Toxoplasma gondii infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J Parasitol 47(2): 125-130.
- Xiao Y, Yin J, Jiang N, Xiang M, Hao L, Lu H, et al. (2010) Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis 10: 4. doi: 10.1186/1471-2334-10-4.
- Kamani J, Mani AU, Egwu GO, Kumshe HA (2009) Seroprevalence of human infection with Toxoplasma gondii and the associated risk factors, in Maiduguri, Borno state, Nigeria. Ann Trop Med Parasitol 103: 317-321.
- Swai ES, Schoonman L (2009) Seroprevalence of Toxoplasma gondii infection amongst residents of Tanga district in north-east Tanzania. Tanzan J Health Res 11: 205-209.
- Silveira C, Belfort R, Jr, Burnier M, Jr, Nussenblatt R (1988) Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am J Ophthalmol 106: 362-364.
- Ybañez RHD, Busmeon CGR, Viernes ARG, Langbid JZ, Nuevarez JP, et al. (2019) Endemicity of Toxoplasma infection and its associated risk factors in Cebu, Philippines. PLoS One 14(6): e0217989.
- Stokkermans TJ, Havens SJ (2023) Toxoplasma Retinochoroiditi.
- Khandwala NS, Hyde RA, Besirli CG (2021) Toxoplasma Retinochoroiditis with Chorioretinal Neovascularization in a Young Patient. Case Rep Ophthalmol 12(1): 259-263.
- Furtado JM, Smith JR, Belfort R, Gattey D, Winthrop KL (2011) Toxoplasmosis: a global threat. J Glob Infect Dis 3(3): 281–284.
- Ozgonul C, Besirli CG (2017) Recent Developments in the Diagnosis and Treatment of Ocular Toxoplasmosis. Ophthalmic Res 57: 1-12.
- Hakim RB, Stewart WF, Canner JK, Tielsch JM (1991) Occupational lead exposure and strabismus in offspring: a case-control study. Am J Epidemiol 133(4): 351-356.
- Kraker J, Zivković SA (2011) Autoimmune neuromuscular disorders. Curr Neuropharmacol 9(3): 400-408.
- Ajamil-Rodanes S, Luis J, Bourkiza R, Girling B, Rees A, et al. (2021) Ocular toxoplasmosis: phenotype differences between toxoplasma IgM positive and IgM negative patients in a large cohort. Br J Ophthalmol 105(2): 210-215.
- Faap M. D. K. W., M.D., B. E., Faap, L. R. M., & Faap, M. M. S. H. (2021) Red Book 2021: Report of the Committee on Infectious Diseases (Thirty-second ed.). American Academy of Pediatrics.
- Roizen N, Kasza K, Karrison T, Mets M, Noble AG, et al. (2006) Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis: implications for compensatory intervention strategies. Pediatrics 118(2): e379-e390.






























