Improving Calcium and Phosphorus Homeostasis in Extremely Premature Infants: A Quality Improvement Project
Sofia M. Markee, DO1*, Janell F. Fuller, MD1, Ann-Marie Yaroslaski, RD, LD2, Eleni E. Shenk, Pharm D3 and Jessie R. Maxwell, MD1,4
1Department of Pediatrics, University of New Mexico, Albuquerque, NM, USA
2Department of Food and Nutrition, University of New Mexico Hospital, Albuquerque, NM, USA
3Department of Inpatient Pharmacy, University of New Mexico Hospital, Albuquerque, NM, USA
4Department of Neurosciences, University of New Mexico, Albuquerque, NM, USA
Submission:December 01, 2022; Published:January 10, 2023
*Corresponding author:Sofia M. Markee, DO, Department of Pediatrics, MSC10 5590, 1 University of New Mexico, Albuquerque, NM. 87131-0001
How to cite this article:Sofia M. Markee, DO, Janell F. Fuller, MD, Ann-Marie Yaroslaski, RD, LD, Eleni E. Shenk, Pharm D, et al. Improving Calcium and Phosphorus Homeostasis in Extremely Premature Infants: A Quality Improvement Project. Acad J Ped Neonatol 2023; 12(2): 555891. 10.19080/AJPN.2023.01.555891
Abstract
Infants born ≤28 weeks are at increased risk for developing electrolyte abnormalities and rely on early parenteral nutrition. Our primary objective was to improve calcium and phosphorus homeostasis in this population by optimizing calcium and phosphorus content in the parenteral nutrition. We completed two PDSA cycles. We collected historical data and analyzed serum calcium and phosphorus levels in the first week of life if obtained during scheduled blood draws (Cohort 1; n=29). We then designed a new, custom parenteral nutrition form for this patient population different to the standardized approach used in the past on all neonatal intensive care unit patients, regardless of gestational age, and analyzed serum calcium and phosphorus levels (Cohort 2; n=9). Realizing that parenteral nutrition is not administered upon admission, our second PDSA cycle consisted of implementing stock IV fluids containing calcium gluconate upon admission (Cohort 3; n=20). Comparisons were made between each Cohort and a mixed-effects model was used. Mean serum calcium and ionized calcium were significantly different between Cohorts, while mean serum phosphorus and serum creatinine were not significantly different. The new, custom parenteral nutrition form and introduction of stock IV fluids containing calcium gluconate on admission allowed a more standardized approach to calcium and phosphorus supplementation aiding in our primary aim of improving calcium and phosphorus homeostasis.
Keywords: electrolyte homeostasis, hypercalcemia, hypophosphatemia, parenteral nutrition
Introduction
Infants born extremely preterm (≤ 28 0/7 weeks gestational age (GA) are at increased risk for developing electrolyte and mineral abnormalities for many reasons, including immature renal function and a reduced baseline bone mineral content [1,2]. Nearly 80% of fetal calcium and phosphorus stores are accreted during the third trimester [2-4]. Missing the third trimester, these infants rely on supplementation from enteral and parenteral sources [2], and as many as 54% of them have been reported to develop metabolic bone disease [4-6].
Metabolic bone disease of prematurity, also known as osteopenia of prematurity, is defined as decreased bone mineral content relative to the expected level of mineralization for a fetus or infant of comparable size or gestational age in conjunction with biochemical and/or radiographic changes [3,5]. The prevalence of metabolic bone disease is inversely associated with birth weight and gestational age, and the etiology is multifactorial, including inadequate postnatal intake of Ca, P and vitamin D, extended periods of total parenteral nutrition, lengthy duration of immobilization and side effects of diuretics and corticosteroids prescribed to these infants [6,7].
The current standard of care is to provide early, aggressive parenteral nutrition (PN) and early enteral feeds to avoid potential metabolic derangements, that could impact growth and neurodevelopmental outcomes [8-10]. PN has been studied to determine optimal dextrose, amino acid and lipid content, but there is limited evidence on how to optimize Ca and P content in extremely premature infants [1]. Additionally, PN has a limited solubility for Ca and P [2].
Hypophosphatemia is one of the first indicators of metabolic bone disease and hypercalcemia is another biochemical change that can occur, along with increased alkaline phosphatase and an elevated tubular reabsorption of phosphorus, all of which indicate disrupted mineral metabolism [3,7]. The disruption of Ca balance from P deficiency is one of the factors leading to hypercalcemia, hypercalciuria, and ultimately nephrocalcinosis [3].
Additionally, acute kidney injury (AKI) is an under-recognized morbidity of extremely low birth weight (ELBW) infants with an estimated 26% prevalence over a 10-year period and a mortality rate more than double that in infants without AKI [11]. AKI in neonates is often multifactorial, but hypercalcemia is one potential insult that can cause nephrocalcinosis, leading to kidney injury and a rise in serum creatinine (SCr). Although SCr is not an ideal biomarker for the early detection of AKI in neonates, small changes in SCr are independently associated with poor outcomes [11].
Materials and Methods
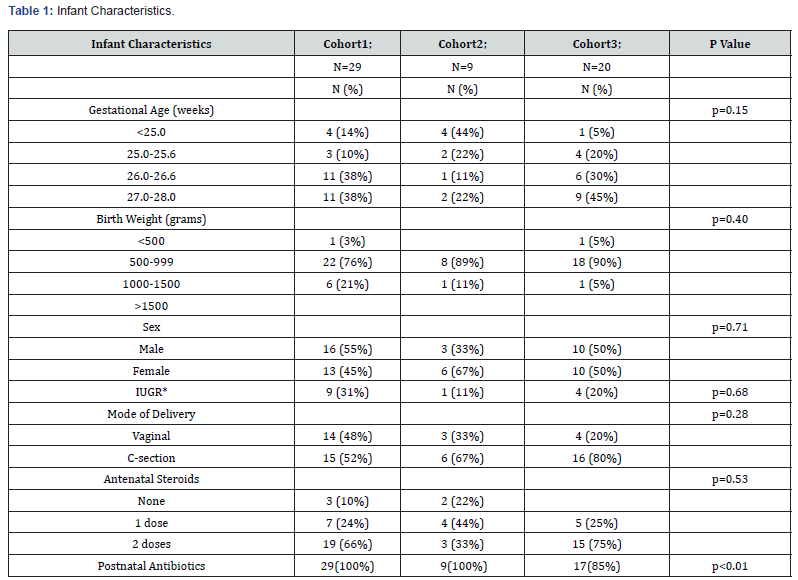
In our level IV neonatal intensive care unit (NICU), we conducted an Institutional Review Board (IRB) approved Quality Improvement project to review and optimize PN content provided to extremely premature infants. Through this process we designed multiple Plan-Do-Study-Act (PDSA) cycles with the aim of optimizing nutrition for bone health and reducing kidney injury in this population. Our primary aim was to improve Ca and P levels over the first week of life in extremely premature infants born ≤ 28 0/7 weeks GA by optimizing PN content. Our secondary aim was to monitor kidney function with SCr, as well as ionized calcium (Ical) levels. We hypothesized that a high proportion of extremely premature infants would have hypercalcemia and hypophosphatemia during the first week of life, leading to increased SCr, our biomarker for kidney injury, with optimization of the PN composition potentially improving Ca and P homeostasis, and preventing elevated SCr levels. We planned a QI project, performing multiple PDSA cycles with the primary objective of improving serum Ca and P concentrations. Electrolyte and PN data in infants ≤ 28 0/7weeks GA were reviewed starting in 2019 via an initial retrospective chart review (historical Cohort 0; n=16). At that time, our standard initial PN solution contained 3 mEq calcium per kilogram and 1.5 mMol phosphate per kilogram for a Ca:P ratio of 2 mEq Ca : 1 mMol phosphate.
We collected data including GA at birth, birth weight, sex, mode of delivery, presence of intrauterine growth restriction, number of doses of antenatal steroids received and type of postnatal antibiotics used in each infant during the first week of life. Beginning in March 2020, we obtained daily serum Ca and P concentrations during the first postnatal week or until full enteral feeds were reached, whichever occurred first (Cohort 1, n=13) at the time of an already scheduled blood draw. Since there was no major change or intervention between Cohorts 0 and 1 other than additional data collection of serum Ca and P in Cohort 1, the decision was made to combine both cohorts and name them Cohort 1; n=29. After reviewing the results of Cohort 1, we designed a new, custom PN for infants ≤ 28 0/7weeks GA. The goal of the new, custom PN was to introduce Ca and P into the IV fluid in a more standardized manner for this specific population. After implementation of the new custom PN formulation consisting of a slow increase in calcium and phosphate and a gradual increase in Ca:P in PN through day of life 7, data was collected on additional infants (Cohort 2, n=9). Our gradual increase in Ca:P consisted of 0.5 mEq : 0 mMol on day of birth, 0.5 mEq : 1 mMol on day of life 1-3, 0.7 mEq : 1 mMol on day of 4, 1.3 mEq : 1 mMol on day of life 5-6, and 2 mEq : 1 mMol on day of life 7.
The next PDSA cycle implemented new stock IV fluids that are commonly utilized in the first 24 hours of life. Prior stock IV fluids used only contained dextrose and amino acids for immediate use following birth, but the new stock IV fluids included calcium gluconate 0.5 mEq/dL (Cohort 3, n=20).
Characteristics of the infants were compared using Fisher’s Exact Test, Student’s t-test, and Chi-Square Test when appropriate. A comparison was made between each cohort. A mixed-effects model with repeated measures was completed in which sphericity was not assumed and Geisser-Greenhouse correction was utilized followed by Tukey’s multiple comparison test. A p-value of < 0.05 was considered statistically significant for all tests. The normal reference ranges used include: serum Ca 6.2-11 mg/dL [12], serum P 5.5-9 mg/dL [3], SCr 0.3-1 mg/dL [12] and iCal 0.9-1.35 mmol/L [13].
Results
A total of 58 infants born ≤ 28 0/7weeks GA were included in our study. No infants were excluded. Baseline infant characteristics are shown in Table 1. The mean gestational age was 26 weeks and 2/7 days and the mean birth weight was 816 grams.
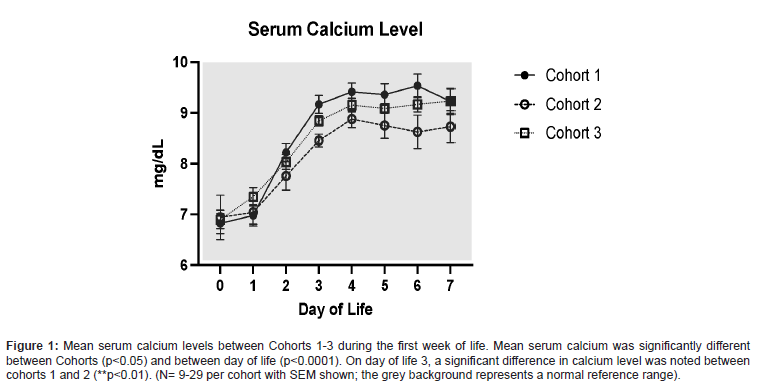
Initial serum Ca levels were low in all cohorts, consistent with the physiologic nadir that is expected within the first 24-30 hours of life [3,14]. Mean serum Ca was significantly different between all cohorts (p<0.05) and between day of life (p<0.0001) (Figure 1). On day of life 0, there was no significant difference noted between any Cohorts, which was reassuring that the cohorts had similar levels at baseline. Additionally, on day of life 1 and 2, there were no differences between Cohorts, likely reflecting continued physiologic levels. However, on day of life 3 a significant difference was noted between cohorts 1 and 2 (p<0.01).
Serum P levels were not observed to have any significant differences between Cohorts (p=0.11) (Figure 2).
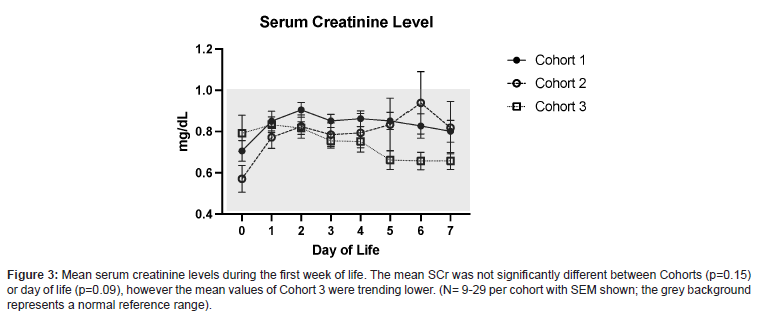
While the mean SCr was not significantly different between Cohorts (p=0.15) or day of life (p=0.09), the mean values of Cohort 3 were trending lower (Figure 3), which could reflect improved kidney function as a result of these interventions.
The mean iCal levels were significantly different between day of life (p<0.0001), but not between Cohorts (p=0.1).
IUGR* – intrauterine growth restriction.


Discussion
The new, custom PN and initial stock IV fluids allowed for a more consistent and gradual increase in serum Ca levels, while remaining within normal limits, during the first postnatal week (Figure 1). Prior to the use of the new PN standardized form for infants born ≤ 28 0/7 GA, the amount of Ca added to PN was not as tightly regulated and led to larger increases in serum Ca (Figure 1), following the physiologic nadir that occurs during the first 24-30 hours of life3. The addition of calcium gluconate 0.5 mEq/dL to stock IV fluids for infants ≤ 28 0/7 GA on DOL 0, as seen in Cohort 3, augmented the improvement and stabilization of serum Ca and SCr levels (Figures 1 and 3). Furthermore, iCal levels remained in goal range, requiring less therapeutic interventions with use of the new PN (Figure 4).
Although we have been unable to show improvement in serum P levels during the first postnatal week of life, low serum P levels during this early time period are consistent with previous reports in this patient population [1,8,9,15-17]. Possible explanations for this include restrictions in the amount of P that can be added to PN due to solubility factors [8], interruption in PN administration due to intolerance or sepsis (such as hypo- or hyperglycemia) in the first week of life [8,15], or the high amino acid intake from early, aggressive PN in extremely preterm infants who are in a state of tissue anabolism, consuming more calories and leading to a higher use of P [8,9,15-18]. We were, however, able to avoid severe hypophosphatemia (serum P level < 1 mg/dL) and resultant hypercalcemia which aided in keeping SCr levels within goal range, hence potentially minimizing the risk of kidney injury from nephrocalcinosis during the first week of life. Additionally, the calcium and phosphorus ratios followed in the new custom PN formulation were more in alignment with recent ESPGHAN guidelines [7,18,19].
Due to the limited sample size, additional studies are needed to determine if the changes implemented truly reflect improvement in kidney function of the extremely preterm neonate, as neonatal SCr levels initially reflect maternal creatinine and take several days to reach equilibrium [11]. In our study, maternal SCr was not routinely measured prior to delivery. We also did not routinely screen neonatal urine calcium to creatinine ratios, which would have been an objective measure of the amount of the Ca excretion in the urine that we could have then compared to the SCr levels.
In conclusion, the use of new stock IV fluids containing calcium gluconate and our custom PN form for infants born ≤ 28 0/7 weeks GA have allowed us to use a more standardized approach to Ca and P supplementation which has aided in the goal to improve outcomes of hypercalcemia, severe hypophosphatemia and SCr levels.
Acknowledgements
The authors would like to acknowledge the contribution and expertise of Jessica Gross, statistician.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boubred F, Herlenius E, Bartocci M, Jonsson B, Vanpée M (2015) Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr 104(11): 1077-1083.
- Körnmann MN, Christmann V, Gradussen CJW, CJW, Rodwell L, Gotthardt M, et al. (2017) Growth and Bone Mineralization of Very Preterm Infants at Term Corrected Age in Relation to Different Nutritional Intakes in the Early Postnatal Period. Nutrients 9(12): 1318.
- Rustico SE, Calabria AC, Garber SJ (2014) Metabolic bone disease of prematurity. J Clin Transl Endocrinol 1(3): 85-91.
- Schulz EV, Wagner CL (2019) History, epidemiology and prevalence of neonatal bone mineral metabolic disorders. Semin Fetal Neonatal Med 25(1): 101069.
- Chacham S, Pasi R, Chegondi M, Ahmad N, Mohanty SB (2020) Metabolic Bone Disease in Premature Neonates: An Unmet Challenge. J Clin Res Pediatr Endocrinol 12(4): 332-339.
- Rehman MU, Narchi H (2015) Metabolic bone disease in the preterm infant: Current state and future directions. World J Methodol 5(3): 115-121.
- Chinoy A, Mughal MZ, Padidela R (2019) Metabolic bone disease of prematurity: causes, recognition, prevention, treatment and long-term consequences. Arch Dis Child Fetal Neonatal Ed. 104(5): F560-F566.
- Brener Dik PH, Galletti MF, Fernández Jonusas SA, Alonso G, Mariani GL, et al. (2015) Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J Perinatol 35(9): 712-715.
- Christmann V, de Grauw AM, Visser R, Matthijsse RP, van Goudoever JB, et al. (2014) Early postnatal calcium and phosphorus metabolism in preterm infants. J Pediatr Gastroenterol Nutr 58(4): 398-403.
- Lucas A, Morley R, Cole TJ (1998) Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ 317(7171): 1481-1487.
- Nada A, Bonachea EM, Askenazi DJ (2017) Acute kidney injury in the fetus and neonate. Semin Fetal Neonatal Med 22(2): 90-97.
- The Harriet Lane Handbook: A Manual for Pediatric House Officers. Twenty-first edition. Philadelphia, PA: Elsevier, 2018.
- Perino JM (2020) Calcium Levels in the Neonate. Neonatal Netw. 2020; 39 (1): 35-39.
- Karpen HE (2018) Mineral Homeostasis and Effects on Bone Mineralization in the Preterm Neonate. Clin Perinatol 45(1): 129-141.
- Bustos Lozano G, Soriano-Ramos M, Pinilla Martín MT, Chumillas Calzada S, García Soria CE, et al. (2019) Early Hypophosphatemia in High-Risk Preterm Infants: Efficacy and Safety of Sodium Glycerophosphate From First Day on Parenteral Nutrition. JPEN J Parenter Enteral Nutr 43(3): 419-425.
- Galletti MF, Brener Dik PH, Fernandez Jonusas SA, Sabatelli D, Chiesa C, et al. (2022) Early high amino-acid intake is associated with hypophosphatemia in preterm infants. J Perinatol 42(8): 1063-1069.
- Tan YL, Tsao PN, Chou HC, Yen TA, Chen CY (2021) Hypophosphatemia as an Early Metabolic Bone Disease Marker in Extremely Low-Birth-Weight Infants After Prolonged Parenteral Nutrition Exposure. JPEN J Parenter Enteral Nutr 45(6): 1268-1274.
- Rizzo V, Capozza M, Panza R, Laforgia N, Baldassarre ME (2022) Macronutrients and Micronutrients in Parenteral Nutrition for Preterm Newborns: A Narrative Review. Nutrients 14(7): 1530.
- Mihatsch W, Fewtrell M, Goulet O, Molgaard C, Picaud JC, et al. (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Calcium, phosphorus and magnesium. Clin Nutr 37(6 Pt B): 2360-2365.






























