Sacrococcygeal Teratoma: A Case Report and Literature Review
Amal Bayar1, Imen Ghadhab1, Yosra Jemaa2*, Rahma Issa3, Haifa Bergaoui1, Amina Mnejja2, Ines Miledi1, Rahma Issa2, Wiem Ben Slamia2, Ines Zouari1, Dhekra Toumi1, Olfa Zoukar1 and Raja Faleh1
1Department of Gynecology and Obstetrics, University Hospital Fattouma Bourguiba, Monastir, Tunisia
2Department of Gynecology and Obstetrics, Hospital HAJ ALI SOUA Ksar Hellel, Monastir, Tunisia
3Department of Gynecology and Obstetrics, University Hospital Taher SFAR Mahdia, Tunisia
Submission: May 10, 2022; Published: June 20, 2022
*Corresponding author:Yosra Jemaa, Department of Gynecology and Obstetrics, Hospital HAJ ALI SOUA Ksar Hellel, Monastir, Tunisia
How to cite this article:Amal B, Imen G, Yosra J, Rahma I, Haifa B, et al. Sacrococcygeal Teratoma: A Case Report and Literature Review. Acad J Ped Neonatol 2022; 11(5): 555879. 10.19080/AJPN.2022.11.555879
Abstract
Sacrococcygeal teratomas are the most common fetal germ cell tumors but remain rare with an incidence of 1 in 35,000 to 40,000 live births. Prenatal ultrasound not only allows for early diagnosis but also for detection of possible complications of these tumors. Management is multidisciplinary. Fetal treatment in utero is now a conceivable alternative thanks to technical progress. We report the case of a sacrococcygeal teratoma which was diagnosed in utero and which was complicated by neonatal death due to the very significant increase in tumor size and the resulting shunt effect.
Keywords: Prenatal; sacrococcygeal teratoma; Complications; Fetal surgery; Endoscopy
Introduction
Sacrococcygeal teratomas represent the most common congenital tumor in newborns and infants, with an incidence of 1 in 35,000 to 40,000 live births [1]. These teratomas account for 40% of all germ cell tumors and 70% of extra gonadal germ cell tumors in the pediatric population [2]. It is a benign disease in 90% of cases, affecting girls 4 times more than boys [3]. Diagnosis is usually made during the second or third trimester of pregnancy by ultrasound, where they appear as a solid, cystic or mixed mass in the sacral region. The prognosis of sacrococcygeal teratomas depends on several factors such as size, tumor growth rate, nature of the predominantly solid mass and tumor vascularity [4]. Perinatal mortality varies between 13% and 50%, and most of these deaths are attributed to heart failure, when the tumor becomes so large during pregnancy, to exsanguinating tumor hemorrhage or both [5].
We report the case of a sacrococcygeal teratoma complicated by heart failure that was diagnosed in utero with ultrasound, with a review of the literature.
Case Report
We report the case of Mrs. L.M, 40 years old, who presented to our emergency room with a threat of severe premature delivery at 29 weeks of amenorrhea (SA). Her history included a spontaneous abortion at 9 weeks of amenorrhea and a full-term delivery by cesarean section for fetomaternal disproportion with a healthy newborn.
The ultrasound examination revealed a sacro-coccygeal mass with exopelvic development, suggestive of a teratoma classified as type I, with poly-lobed contours, heterogeneous with a predominantly solid component measuring 18 cm in diameter. In addition, the presence of associated hydramnios and the absence of hyperemia or shunt effect on Doppler examination were noted.
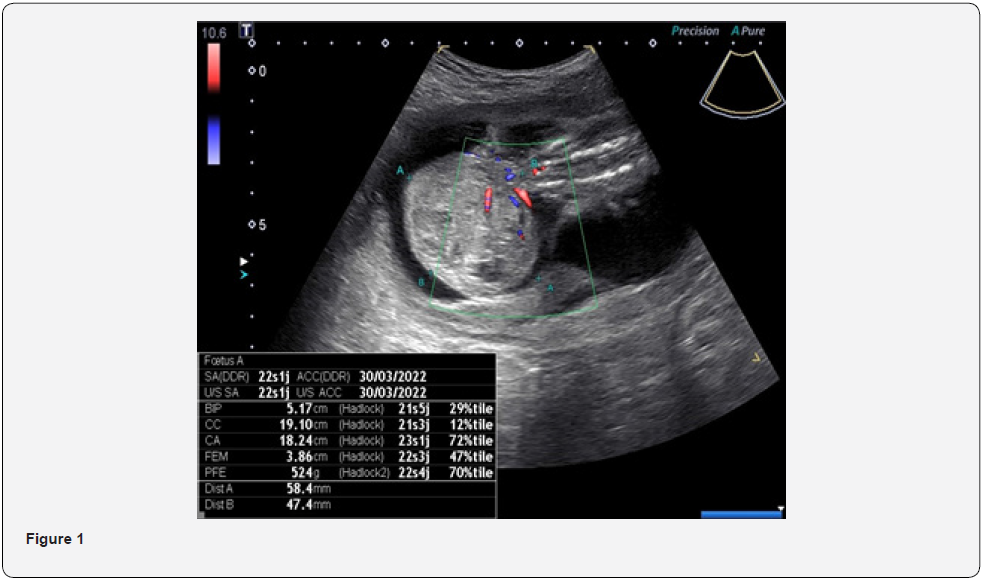
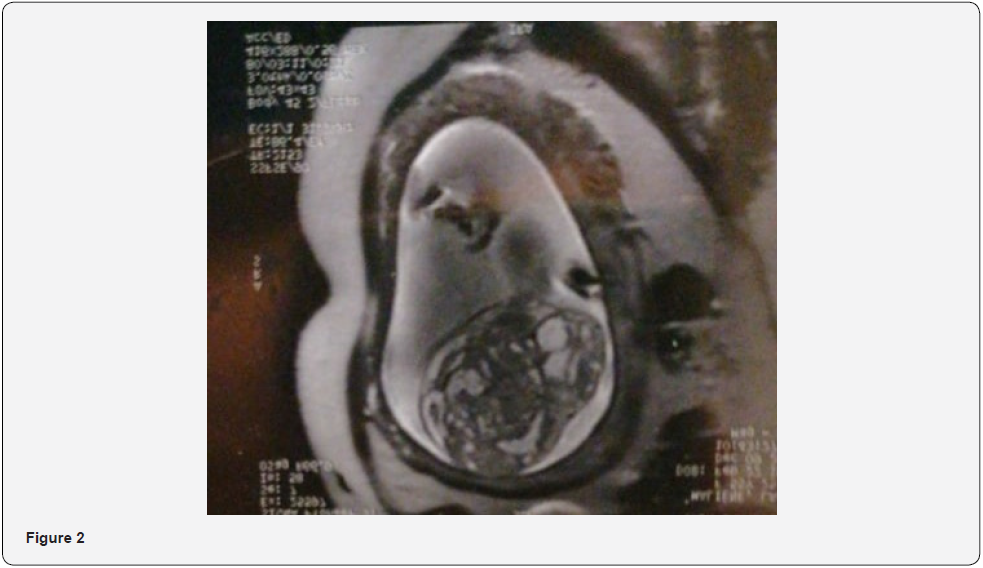
The diagnosis of sacrococcygeal teratoma was made at 22 weeks’ gestation during the morphological ultrasound, where it measured 47x58mm (Figure 1). Her gynecologist asked her for a complementary exploration through magnetic resonance imaging (MRI) which better studied the lumbar spine to confirm its integrity with the absence of associated anomalies (Figure 2).
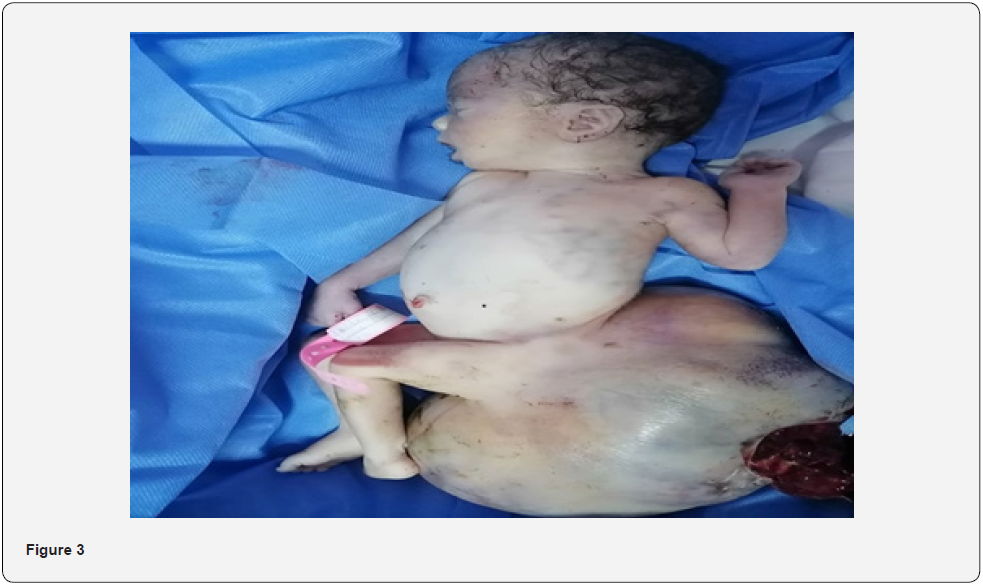
The acute episode of threatened preterm delivery was controlled by tocolysis with the administration of a corticosteroid pulmonary maturation cure. Close fetal monitoring (especially echocardiographic) was organized during the multidisciplinary decision. The evolution was marked by the development of maternal fever, which was due to COVID-19 infection, further complicating the management. During fetal heart rate monitoring,repetitive decelerations were noted attesting to a state of fetal suffering at 31 SA. An emergency cesarean section was indicated. The extraction was dystocic because of the sacro-coccygeal teratoma, which was 30 cm long, and the baby, male, was in an apparent dead state (Figure 3). Immediate resuscitation of the neonate was done but he did not recover.
Discussion
Fetal teratomas are rare. The reported incidence is between 0.07 and 2.8 per 1000 pregnancies [6]. Approximately 90% of human teratomas develop in the gonads, mostly in the testes, but a few cases develop in extra-gonadal sites such as the brain, neck, mediastinum, retroperitoneum, and sacro-coccygeal area, which remains the primary site of development of extra-gonadal teratomas in newborns and young infants [7]. CST develops from the sacrum and coccyx, protruding outward and increasingly into the pelvic cavity. It develops from the remnants of the primitive streak that normally degenerates and disappears around the 4th week of development. It is derived from pluripotent cells of the three germ cell layers (entoderm, ectoderm and mesoderm) from the primitive streak, resulting in a formation that is often made up of different tissues (bone, hair, teeth, nerves...) [8]. Sacrococcygeal teratomas occur sporadically and no genetic or chromosomal link has been established [9]. Therefore, the indication for amniocentesis for fetal karyotype is not recommended in simple forms of SCT. However, associated structural abnormalities may be found in 11% to 38% and may include sacral and spinal anomalies and concomitant anorectal malformations [10].
In 1974, ALTMAN et al [10] proposed a topographic classification of sacrococcygeal teratomas in their report to the American Academy of Pediatric Surgery:
Type I: type I teratomas are almost exclusively external with a minimal pelvic component.
Type II: Type II teratomas have a significant pelvic component.
Type III: the intra-abdominal and intra-pelvic component is much greater than the external component.
Type IV: They are exclusively pre-sacral with no external component; this is the most difficult form to diagnose both clinically and on ante- or postnatal ultrasound.
Large TSS can exert a mass effect on the intra-pelvic organs and present serious consequences such as constipation, fecal incontinence, neurogenic bladder, and urinary incontinence.
The generalization of prenatal ultrasound has made it possible to diagnose sacrococcygeal teratomas as early as intrauterine life, and this can be very early in the first trimester of pregnancy [11]. Indeed, in the two series of Tongson et al and Ho et al [12], the antenatal diagnosis was made from the 13th week of amenorrhea. The use of ultrasound makes it possible to limit the formation in question, to specify its solid or cystic nature, to detect complications such as intra-tumoral haemorrhage or necrosis and to look for associated malformations. It also allows close monitoring of pregnancy, tumor size, and assessment of tumor vascularity using Doppler flow. This is due to the fact that tumors, richly vascularized and presenting a rapid growth or having a solid predominance, tend to undergo the phenomenon of vascular flight which exposes the fetus to a high risk of developing hydrops or feto-placental hydrops with the consequence of maternal pseudo pre-eclampsia or the mirror syndrome [13]. For high-risk tumors, the frequency of surveillance is set at twice a week; for low-risk tumors, respectively small, cystic, or with decreased vascularity, the frequency can be reduced to once every two weeks.
Ultrasound is of great help in sacrococcygeal teratomas, but it is sometimes limited and not very sensitive, especially when compared with magnetic resonance imaging (MRI). Indeed, MRI is an excellent tool to evaluate the exact size of the tumor, its content and its extension to the pelvic organs to optimize the pre and post-natal management. It also allows, thanks to its good sensitivity when imaging the lower spine, to search for associated malformations such as hip dislocation and spinal dysraphism [14].
Because of the fear of possible complications of sacrococcygeal teratomas, the possibility of fetal death and maternal consequences in hypervascularized forms, therapists are increasingly moving toward a more interventional approach than simple prenatal monitoring. This in utero therapeutic approach aims either at the removal of the tumor or at reducing its vascularization and thus decreasing its growth potential; which allows to diminish the burden of the SCT on the fetal cardiovascular system [2]. Open uterus” fetal surgery allows access to the fetus through a maternal laparotomy with hysterotomy [15]. The major problem, and the real Achilles’ heel of this therapeutic approach, is significant maternal and fetal morbidity. Hence the role of fetal endoscopy, which has proven its effectiveness with fewer complications. Minimally invasive procedures used for the management of complicated sacrococcygeal teratomas include radiofrequency ablation, YAG laser ablation, ultrasound-guided percutaneous puncture-drainage, and thermoablation. Researchers in Toronto [16] tried laser ablation, radiofrequency ablation and intravascular embolization in five fetuses with CST; only two patients survived after these procedures. Other experiences from Brazil and France have shown similar results of percutaneous interventions [17]. Therefore, fetal treatments of SCT, surgical or interventional, are still experimental. However, given the poor prognosis of fetuses developing heart failure and hydrops in utero, these early intervention techniques deserve to be investigated and further developed.
Conclusion
CSTs are rare congenital tumors with high risk of perinatal morbidity and mortality. Ultrasound remains the technique of choice to make an early diagnosis and establish a therapeutic regimen. As soon as the diagnosis of CST is made, parents must be informed and given psychological support. The management of this tumor is multidisciplinary. Endoscopic interventions have made considerable progress, allowing nowadays to address serious fetal pathologies, formerly lethal, but remain to be improved.
Consentement
Informed consent for the publication of their clinical details and/or clinical images has been obtained from the parents
Conflict of Interest
The authors declare no conflict of interest.
Contribution of the Author’s
All authors also declare that they have read and approved the final version of the manuscript.
References
- Kamal NR, Jasbir S (2021) Neonatal sacrococcygeal teratoma: Our 20-year experience from a tertiary care centre in North India. Tropical Doctor. Sage J Vol 51(2): 209-212.
- Bianca MM, Roxana EB, Vlad D, Veduta A, Georgescu TA, et al. (2021) Update in the prenatal management of sacrococcygeal teratomas and the outcome of the newborn: case report. Ro J Pediatr 70(3): 192-196.
- Akinkuotu AC, Coleman A, Shue E, Sheikh F, Hirose S, et al. (2015) Predictors of poor prognosis in prenatally diagnosed sacrococcygeal teratoma: a multiinstitutional review. J Pediatr Surg 50(5): 771-774.
- Usui N, Kitano Y, Sago H, Kanamori Y, Yoneda A, et al. (2012) Outcomes of prenatally diagnosed sacrococcygeal teratomas: the results of a Japanese nationwide survey. J Pediatr Surg 47(3): 441-447.
- Kamil D, Tepelmann J, Berg C, Heep A, Axt-Fliedner R, et al. (2008) Spectrum and outcome of prenatally diagnosed fetal tumors. Ultrasound Obstet Gynecol 31(3): 296-302.
- Barksdale EM, Obokhare I (2009) Teratomas in infants and children. Curr Opin Pediatr 21(3): 344-349.
- Isaacs H (2004) Perinatal (fetal and neonatal) germ cell tumors. J Pediatr Surg 39(7): 1003-1013.
- Lubala TK, Mukuku O, Shongo MP, AM Mutombo, Lubala N, et al. (2015) Sacrococcygeal teratoma in a female newborn with clinical features of trisomy 13: a case report from Central Africa. Int Med Case Rep J 8: 333-336.
- Gupta R, Sharma SB, Mathur P, Goyal RB (2014) Variants of Currarino syndrome: embryological association and review of pertinent literature. Int J Embryol 2014: 1-5.
- Ji Hoon Phi (2021) Sacrococcygeal Teratoma: A Tumor at the Center of Embryogenesis. J Korean Neurosurg Soc 64(3): 406-413.
- Lee MY, Won HS, Hyun MK, Hee-Young Lee, Jae-Yoon Shim, et al. (2011) Perinatal outcome of sacrococcygeal teratoma. Prenat Diagn 31(13): 1217–1221.
- Imane El ANTAKI (2017) Sacrococcygeal teratomas in children in the department of pediatric surgery B (About 16 cases). Faculty of Medicine and Pharmacy Marrakech Thesis No. 05.
- Wohlmuth C, Bergh E, Bell C, Anthony Johnson, Kenneth j Moise, et al. (2019) Clinical monitoring of sacrococcygeal teratoma. Fetal Diagn Ther 46(5): 333-340.
- Kremer MEB, Althof JF, Derikx JPM, Robertine van Baren, Hugo A Heij, et al. (2018) The incidence of associated abnormalities in patients with sacrococcygeal teratoma. J Pediatr Surg 53(10): 1918-1922.
- Graesslin O, Martin-Morille C, Dedecker F, R Gabriel, C Quereux, et al. (2004) Tératomes sacrococcygiens. Y a-t-il une place pour le traitement in utero des formes compliquées : À propos de trois cas. Gynécologie Obstétrique et Fertilité 32(6): 519-524.
- Van Mieghem T, Al-Ibrahim A, Deprest J, L Lewi, JC Langer, et al. (2014) Minimally invasive therapy for fetal sacrococcygeal teratoma: case series and systematic review of the literature. Ultrasound Obstet Gynecol 43(6): 611-619.
- Sananes N, Javadian P, Schwach WBI, N Meyer, A Koch, et al. (2016) Technical aspects and effectiveness of percutaneous fetal therapies for large sacrococcygeal teratomas: cohort study and literature review. Ultrasound Obstet Gynecol 47(6): 712-719.






























