State Academic Consensus for the Correct Use of Antibiotics in Newborns
Federico Javier Ortiz-Ibarra1*, Jesús Reyna-Figueroa2*, Cesar Humberto Botello-Ortiz3, Laura Mejía-Caballero1, Alberto Carrillo-González1, Darinka Neyvi López-Carbajal3, Mariel Garrido-Vázquez3, Enrique Rafael Ortiz -García3, Brandon Ortiz-Casas4 Patricia Saltigeral-Simental5, Gabriel Lara-Flores6, Sergio G. Golombek7 and Francisco Javier Fernández-Clamont9
1Maternal and Perinatal Hospital “Mónica Pretelini Sáenz”. Institute of Health of the State of Mexico. Toluca, State of Mexico, Mexico
2Institute of Health of the State of Mexico. Toluca, State of Mexico, Mexico
3Mother-Child Institute of the State of Mexico. Toluca, State of Mexico, Mexico
4General Hospital “Nicolas San Juan” , Toluca, State of Mexico
5Monterrey Institute of Technology, Mexico City, Mexico
6National Institute of Pediatrics. Mexico City, Mexico
7Association of Neonatologists of Mexico City and the Valley of Mexico. Mexico City, Mexico.
8SUNY Downstate Health Sciences University, Brooklyn, New York, US
9Secretary of Health, Institute of Health of the State of Mexico. Toluca, State of Mexico, Mexico
Submission: May 10, 2022; Published: June 20, 2022
*Corresponding author:Federico Javier Ortiz-Ibarra, Maternal and Perinatal Hospital “Mónica Pretelini Sáenz”. Institute of Health of the State of Mexico. Toluca, State of Mexico, Mexico, Tel: +52 1 5539881069
Jesús Reyna-Figueroa, Institute of Health of the State of Mexico. Toluca, State of Mexico, Mexico
*Collaborative group
Gabriela García-Cuevas, Tariacuri Aguilar-Magaña, Erika Miraflores-Vidaurri. Yair Gerardo Navarrete Ávila: Maternal and Perinatal Hospital “Mónica Pretelini Sáenz”. Toluca, State of Mexico
María Estela Ramírez-San Martin, María Eugenia Pacheco-Bárcenas, Erika García- Carrillo. Elliot Ortiz Hernández: General Hospital Maximiliano Ruiz Castañeda, Naucalpan, State of Mexico.
Genaro Sabais-Herrera: Las Americas General Hospital, Ecatepec, State of Mexico.
María Bertha Romo Almanza: Maternal Hospital Guadalupe Victoria, Atizapán, State of Mexico.
Delia Margarita Velarde-Rojas: General Hospital Josefa Ortiz de Dominguez, Chalco, State of Mexico.
Remedios Hernández Pallares, Octavio Rafael Mancilla Sánchez: General Hospital José Vicente Villada, Cuautitlán, State of Mexico
Reyna Arellano Arroyo: General Hospital Nicolas San Juan, Toluca, State of Mexico
Juan Carlos González-Sánchez: Children’s Hospital Maternal and Child Institute of the State of Mexico
How to cite this article:Federico J O-I, Jesús R-F, Cesar H B-O, Laura M-C, A C-G, et al. State Academic Consensus for the Correct Use of Antibiotics in Newborns. Acad J Ped Neonatol 2022; 11(5): 555876. 10.19080/AJPN.2022.11.555876
Abstract
Introduction: The use of antibiotics in newborns continues to be an area of opportunity to improve the standards of healthcare in the medical field.
Objective: To avoid the abuse of antibiotics in newborns treated in the hospital network of the Institute of Health of the State of Mexico, an Academic Consensus was carried out to propose, based on the best scientific evidence, the state of recommendations on prudent and rational use of antibiotics.
Methodology: The academic consensus model was used, following the recommendations of the Guidelines for the elaboration of consensus and the recommendations and useful criteria for the critical reading of selected articles of the European initiative AGREE. For the formation of the participating group, mixed and representative teams were formed, in which neonatologists, pediatric infectious diseases specialists, and pharmacists responsible for the pharmacovigilance of participating hospitals were considered.
Results: The members of the working groups of the consensus selected a total of 10 questions that still present controversy or confusion about the use of antibiotics in newborns. These questions were deemed to seek, under the best current evidence, the most appropriate recommendations for the rational and prudent administration of antibiotics and antifungals in newborns. The results of each of the analyzes, as well as the conclusions and recommendations are presented with their respective degree of recommendation and level of evidence.
Conclusion: The consensus group concludes that the evidence found and presented as a set of recommendations for clinical applications will favor reducing empirical practice and great variability in the use of broad-spectrum antibiotics in neonatal care medical units (NICUs). Further, it will also benefit the process of standardization through their integration into a manual for the use of antimicrobials procedure standards for the NICU.
Keywords: Neonatal sepsis, antibiotics, antifungals, neonates
Introduction
The use of antibiotics in newborns continues to have a plethora of opportunities to improve the quality of medical standards worldwide. Since the 60s, some leading figures such as Dr. James Sutherland considered it one of the areas with the highest level of clinical ignorance, due to its empirical approaches, and the lack of groundbreaking studies beyond the “heroic” years of neonatology. His clinical acuity allowed him to document severe adverse effects associated with the use of antimicrobials in this age group, such as the case with novobiocin and its association with hyperbilirubinemia in nurseries, as well as the case of chloramphenicol with fetal cardiovascular collapse [1,2].
Afterwards, several dozen antibiotics have been used in newborns, under off-label justification, in critical situations, and long before controlled clinical trials were presented to minimize the risk of adverse effects.
Despite the great efforts worldwide to promote rational use of antibiotics in newborns, their loose and unwise usage continues to be a constant in many neonatal units. In the review presented by Cardetti, there is an insistence on the risks of possible negative effects in the short and long terms of the use of antibiotics during the neonatal period, highlighting the presence of systemic candidiasis, the increase in necrotizing enterocolitis (NEC) alterations of the intestinal microbiome, late-onset sepsis, the appearance of multi-resistant microorganisms, as well as the increase in pediatric mortality of patients who receive multiple antibiotic regimens in the neonatal stage [3].
To standardize better medical practices in hospitals with neonatal care within the hospital network of the Institute of Health of the State of Mexico (ISEM, due to its acronym in Spanish), and as one of the quality policies generated by its authorities, a medical group conformed by neonatologists, pediatric infectious diseases specialists, and pharmacists from the Institute’s NICUs reached a consensus for the proper and prudent use of antibiotics in newborns with severe infections, which allowed the recommendations on prudent and rational use of antibiotics based on the best evidence. For the latter, an academic consensus was organized, where all the conclusions and recommendations were presented according to their level of evidence and degree of recommendation.
Methodology
The academic consensus model used consisted of the analysis and discussion of the available scientific literature, which was further complemented with the review, opinion, and analysis from experts within the area on topics considered controversial, or whose bibliographic evidence was considered insufficient.
We followed the recommendation of the guide «Lineamientos para la elaboración de eve lof» (Guidelines for the elaboration of consensus) [4] and the recommendations and useful criteria for the critical reading of selected articles of the European initiative AGREE (Appraisal of Guidelines for Research & Evaluation) [5].
Mixed and representative teams were formed, with neonatologists, pediatric infectious diseases specialists, and pharmacists responsible for the pharmacovigilance of participating hospitals were invited.
The group coordinators established a selective search strategy for articles in which clinical studies, reviews, and previous consensus were obtained and further analyzed.
The selection of articles was carried out using the following keywords: neonatal sepsis, antibiotics in the neonate, neonatal infection, antibiotics in the newborn, antimicrobials and the newborn, among synonymous terms. Articles only within the period from January 2010 to December 2020 were included. Articles published in both English and Spanish in the PubMed, Google Scholar, EMBASE, and Cochrane search engines were selected. As mentioned, original articles and related reviews were included, as well as consensus and opinion letters made by experts in the field. Additionally, ascending searches were realized using some of the references of the selected articles, especially in the analysis of long-term follow-up studies.
Once the total amount of bibliography was identified, a first virtual meeting was held, where the scope for the topics to be analyzed was defined. In this same meeting, the bases were established for the adoption of recommendations from other organizations or societies to be included in the conclusions of this consensus. With the aforementioned, four working tables were designed with two coordinators per group.
The second phase was also carried out remotely in meetings on the official ISEM platform, as well as in daily contact in meeting groups (chats) formed on the WhatsApp platform between the participating groups and the consensus coordinators. After the phase of critical review of the literature, and with the results and conclusions obtained by each group, the consensus coordinators wrote the first answers to the selected questions, which were presented to the group during the virtual meetings as a first draft, which was subsequently reviewed by the entire group. The validation of the manuscript was carried out by three external parties, who carried out an independent review. Their observations and recommendations aided in the reformulation of the consensus questions and were integrated into the final document.
Consequently, all the conclusions and recommendations are presented with their levels of evidence, degree of recommendation, and hierarchical level of evidence, as well as their proposal for applicability in clinical practice [5,6].
Tables 1 and 2 present the scales of the Canadian Task Force on Preventive Health Care (CTFPHC), developed by the Public Health Agency of Canada (PHAC) to develop clinical practice guidelines that support the selected preventive health actions. These are used for the evaluation of the degree of recommendation and level of evidence of issued health recommendations [6,7].
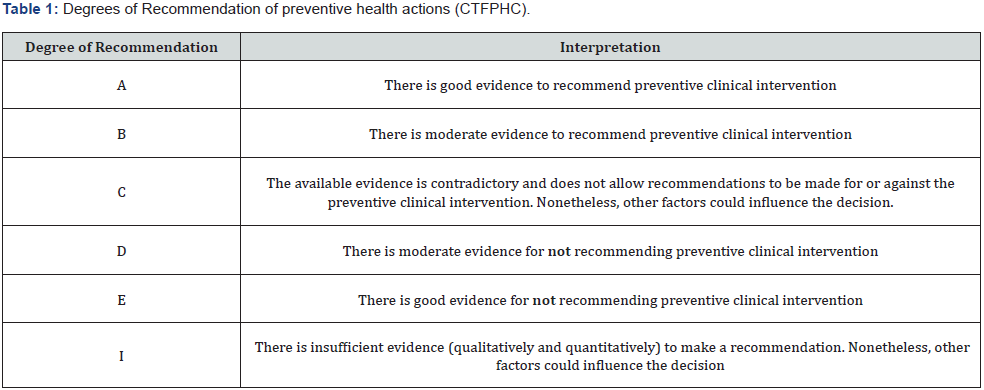
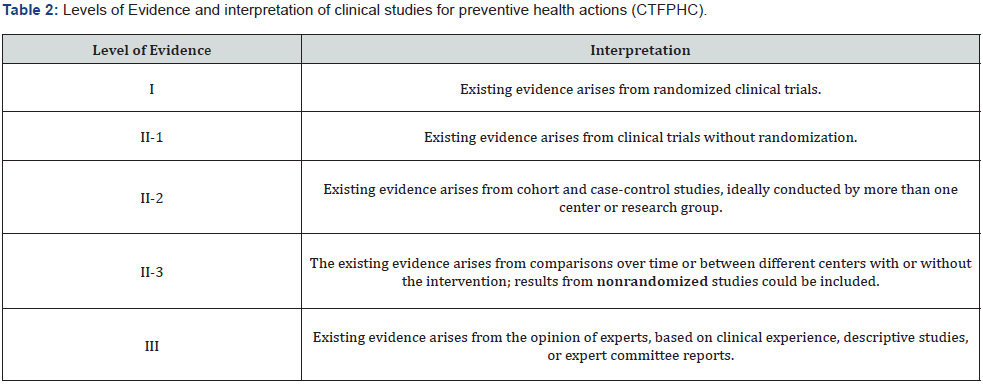
Based on these guidelines, the members of the working groups of the consensus selected a total of 10 questions that were believed to present controversy or confusion within the use of antibiotics in newborns. These questions were constituted to seek, under the best current evidence, the most appropriate recommendations for the rational and prudent administration of antibiotics and antifungals in newborns.
Results
Question 1: What definition is generally accepted for the denomination of the neonate with sepsis?
Reviewing the last 10 years to date, a universal consensus has not been reached to clearly define neonatal sepsis. In fact, various authors proclaim that it is necessary to establish a real consensus to achieve this purpose [8-13].
As described by Reyna et al. [14], the definitions used in different consensuses, guides, and review articles continue to be very heterogeneous and most of the lack academic validation.
The consensus definitions used in older children in 2005, and 2016 [15,16] have been shown to have low diagnostic performance for the integration of the neonate with sepsis, with assertiveness percentages between 42-56% in early neonatal sepsis and being as low as 8.9% in the premature newborn [17].
The use of the criterion of having organ dysfunction has shown a sensitivity of less than 50% of neonates with sepsis who had organ dysfunction, only 56% met the criteria for systemic inflammatory response syndrome (SIRS) [18].
Most of the review articles and expert opinions highlight the importance of establishing a universal definition of neonatal sepsis, but few raise a proposal.
Wynn and Polín’s proposal [10] to have a static definition of sepsis has potential advantages, but it also has limitations, which include the inability to incorporate changes in the condition over time. This represents a limitation, since sepsis is a dynamic, complex process with a heterogeneous condition. Moreover, even though the clinic has not shown much diagnostic value, some technological advances within the field of molecular biology testing might bring us closer to a more accurate diagnosis.
For the purposes of having a regionalized definition, it is proposed to return to one of the simplest definitions that this entity has had, but that integrates the clinical and laboratory spectrum, as well as its causal association with an infectious agent, and based on this review:
Neonatal sepsis is a syndrome with altered clinical and laboratory data (Table 3) [19-24], presented in the first 30 days of life, caused by the system invasion of an infectious agent, (bacteria, virus, or fungus), which can lead to one or more organ failures. Level of evidence III, Level of recommendation C.
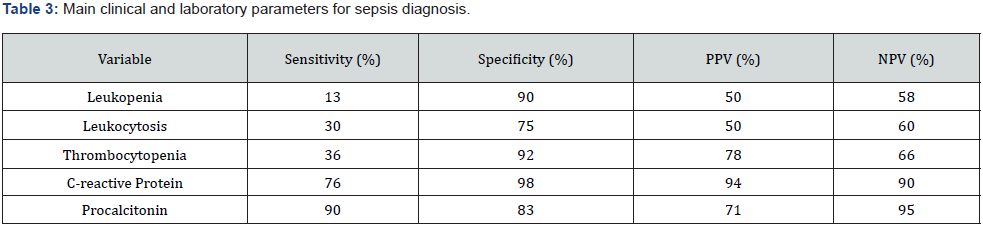
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of some laboratory parameters in neonatal sepsis [19- 24].
But like many of these authors, the consensus established that this definition must be validated with an adequate academic process.
Question 2: What is the value of the clinical and laboratory spectrum count for the diagnosis of neonatal sepsis?
There are several clinical manifestations that have been described in neonatal sepsis. However, each of these cases alone has a variable prevalence and a low positive predictive value, usually below 20%.
Husada’s case-control study, which included 52 neonates with confirmed sepsis and 156 controls, found that the four clinical variables with the highest predictive level for neonatal sepsis were: poor feeding, heart rate changes, dysthermia, and saturation <92% [25].
Another cohort study by Das et al. [26] that included 279 very low birth weight preterm infants with late-onset sepsis found that hypothermia and apnea were significantly associated with culture-proven sepsis (p=0.015 y 0.004, respectively) [26]
Kayange’s prospective study evaluated 300 newborns with early and late sepsis and studied clinical predictors of sepsis with a positive culture. For early sepsis, the predictors were: tachypnea, lethargy, poor feeding, cyanosis, and seizures; while for late sepsis the predictors were: changes in temperature (hyperthermia or hypothermia), lethargy, poor feeding, cyanosis, jaundice, and seizures [27].
A prospective study by Weber studied the clinical predictors of severity in both early and late neonatal sepsis, including newborns in developing countries, finding in a multivariate analysis that the independent predictors of disease severity were: fever, low feeding intake, hypoactivity (or decreased spontaneous movement),lethargy, changes in activity, feeding problems, agitation, chest retractions, grunting, cyanosis, respiratory rate >60%, history of seizures, bulging fontanelle, and prolonged capillary refill. In that same article, a sensitivity of 87% was determined by the presence of one of the 14 mentioned predictors, and a specificity of 54%. By including two of the predictors, the sensitivity dropped to 69% and the specificity increased to 77% [28].
A meta-analysis published in 2015 by Verstraete evaluated different clinical prediction models published, specifically for neonatal healthcare-associated infections (HAIs) which represented 1295 suspected and 434 confirmed cases, finding that, although most of the models had good methodological quality, their diagnostic accuracy was low. In that same study, post hoc analysis found that apnea, bradycardia, lethargy, reticulated or mottled skin, and poor distal perfusion had statistically significant predictive value for neonatal sepsis associated with health care. This metanalysis was limited due to the clinical and statistical heterogenicity of the articles included [29].
addition to clinical criteria, there are various risk factors associated with early neonatal sepsis, which can be taken into account and related to clinical data for presumptive diagnosis. In 1999, Benitz and collaborators studied the main risk factors associated with early sepsis, finding that maternal colonization by group B Streptococcus (OR=9.64), weight below 2500g (OR=7.37), less than 28 weeks of gestation (OR=21.7), membrane rupture greater than 18h (OR=7.28), and maternal temperature >37.5 °C during labor (OR=4.05) were the factors with a significant relationship [30].
In a case-control study conducted by Masanja, risk factors for neonatal sepsis were evaluated. The maternal factors that showed significant association with early sepsis were: Chorioamnionitis (OR=2.9), and premature rupture of membranes (OR=1.91).
Perinatal asphyxia was shown to be a neonatal factor significantly associated with early sepsis (OR=6.7) [31].
To have greater diagnostic precision, algorithms and calculators have been developed, such as the Kaiser Permanente Early-onset Sepsis Calculator [32], which integrates maternal risk factors, the incidence of neonatal sepsis in the region, and the clinical status of the newborn; thus, calculating the probability of neonatal sepsis and suggesting the type of intervention that can be: 1) No intervention; 2) Monitor vital signs without culture and without starting antibiotics; 3) Monitor vitals, culture, but do not start antibiotics; and 4) Start antibiotics.
The use of the sepsis calculator has been shown to reduce the number of interventions and the use of antibiotics in newborns with risk factors for sepsis, without increasing the risk or mortality in these patients [32-34].
A systematic review and meta-analysis by Deshmuket et al. [35] reviewed a total of 387 articles. This meta-analysis (random effects model) showed that implementation of thr sepsis calculator was associated with reduced antibiotic usage (N = 172,385; OR = 0.22 (0.14–0.36); p < .00001; heterogeneity (I2) = 97%), Number needed to treat (NNT)=22, laboratory tests (N = 168,432; OR = 0.14 (0.08–0.27); p < .00001; I2 = 99%, NNT = 8), and admissions to neonatal unit (N = 16,628; OR = 0.24 (0.11–0.51); p = .0002; I2 = 98%, NNT = 7); LOE: moderate. There was no difference in mortality, culture positive EOS, and readmissions [35].
Another meta-analysis published in 2019 by Achten and colleagues, included 13 pre- and post-intervention studies (with a total of 175,752 newborns). A statistically significant reduction in antibiotic use was found in neonates using the sepsis calculator. The evidence to assess the safety of the calculator’s implementation was limited, although, the proportion of underdiagnosed neonatal sepsis cases were similar in the calculator and non-calculator groups [36].
Consensus Recommendations
The clinical data of neonatal sepsis alone have a low positive predictive level, so the patient’s risk factors must be combined with the clinical features for decision-making in the diagnosis of sepsis in the newborn. (Level of evidence II-1 Degree of recommendation A)
Consider the following as the most important maternal risk factors for early neonatal sepsis: maternal chorioamnionitis, membrane rupture greater than 18 hours, maternal fever during labor, and colonization by Streptococcus agalactiae (Level of evidence II-1, Degree of recommendation A)
Consider the following clinical indicators highly suggestive indicators of late sepsis: Fever or hypothermia, poor feeding, changes in activity level, mottled or reticulated skin, prolonged capillary refill (+2 seconds), and bradycardia (unexplained by another cause / non-infectious) (Level of evidence II-2, Degree of recommendation A)
If a newborn with risk factors is asymptomatic, starting with antibiotic treatment is not recommended. (Level of evidence II- 2, Degree of recommendation A)
It is recommended to implement in neonatal care units the use of the neonatal sepsis calculator, with the intention of reducing the overuse of antibiotics in newborns. (Level of evidence I, Degree of recommendation A)
Question 3: Which microbiological diagnostic study can achieve a higher degree of diagnostic certainty of neonatal sepsis in our hospital environment?
As already mentioned, there is no clinical pattern that can be assertive on its own for the diagnosis of sepsis, so laboratory diagnostic tools continue to be the best alternative for the adequate diagnosis of this syndrome.
Regarding blood cultures, although they have been considered the gold standard for proven neonatal sepsis, its diagnostic yield is low or very low, (0.4-0.8/ 1000 sampled newborns). In a systematic review of neonatal sepsis, variable correlations (although all low) were found between corroborated sepsis with cultures vs sepsis with negative cultures (1:6 – 1:16), which is equivalent to a variable frequency of positives between 5.9% - 14.7% [37]. To increase the diagnostic performance of blood cultures, it is important to obtain a proper sampling. Schelonka et al. [38] studied the sensitivity of blood cultures to detect common pathogens in neonatal sepsis with a concentration of 1 – 10 UFC/ mL. The probability of isolating the pathogen with 0.5mL of blood decreased from concentrations lower than 4 CFU/mL, compared to 1 mL or more of the sample [38].
In a prospective controlled study, the positivity of blood cultures in newborns with true bacteremia was compared with a single 1 mL sample inoculated in the same flask, vs. dividing the sample into two 0.5 mL samples inoculated each in different bottles (aerobic and anaerobic). Microbiological isolation was significantly higher in the control group of two 0.5 mL samples in two different culture media, than in the control group with 1 mL sample and one culture medium (94.4% vs 77.7%, P= 0.012) [39].
Another prospective cohort study compared the efficacy of taking two blood cultures from different sites vs. one blood cultures in neonatal sepsis diagnostics. A higher positivity rate was found in the group of two blood cultures, compared to that of one blood culture (46.5% vs 38.9%, P=0.018). A percent positivity difference of 7.6% was found [40].
However, in favor of this technology, much of the microbiological recovery will depend on the recovery capacity of the equipment used and whether or not the media have elements capable of inhibiting possible antibiotics circulating in the blood, as demonstrated in a study controlled in vitro using the BactAlert 3D® and media with antibiotic inactivation resins, where it was determined that this automated system detect six microorganisms commonly found in neonates, with volumes from 0.3 mL of blood and minimum concentrations of microorganisms (1-10 CFU/mL) [41].
On the other hand, molecular systems for the diagnosis of infections have become a fundamental part of clinical diagnostics, starting to replace international gold standards more and more frequently. For the latter, neonatal sepsis has not been an exception. Today, various platforms dependent on genetic material such as Polymerase Chain Reaction-based and Sequencing-based technologies offer great advantages of diagnostic accuracy and less time, improving diagnostic sensitivities and specificities in this global health issue [42,43].
Meningitis in cases of early sepsis is rare, being more frequently found in late neonatal sepsis. The incidence of meningitis is 0.2- 0.3% live births, but in preterm newborns is 0.5-14%.
Lumbar puncture (LP), as a microbiological recovery technique, has been decreasing in use in recent years in Mexican protocols; perhaps due to the influence of the early use of broad-spectrum antibiotics that apparently have neglected the presentation of early meningeal symptoms.
An LP is essential for the diagnosis of meningitis but is not recommended in suspected early neonatal sepsis. It is recommended to perform an LP in neonates older than 72 hours from birth, symptomatic, and with suspected late sepsis, although the incidence is low (between 1.5% y 3.5%).
To support or not its use, a retrospective study was evaluated, which included patients with suspected neonatal sepsis where only 11 of 1712 (0.6%) of the symptomatic patients had meningitis. From another group with a total of 3423 asymptomatic babies, none of them (0%) developed meningeal infections [44]. Another study in preterm infants (between 27-36 gestation weeks) diagnosed with respiratory distress syndrome, in whom LP was performed routinely, only 0.3% had meningitis [45]. Similar results were observed in the retrospective study by Smith, which included 4,632 neonates with suspected sepsis in whom LP was performed. Only 2% had meningitis [46].
A different eve lofn is observed with microorganisms such as Streptococcus agalatie or Listeria monocytogenes, where late presentations of the disease can show higher recovery rates, as demonstrated in the Ansong study where 13,495 neonates underwent LP in the first 7 days of life, 14% of neonates with Group B Streptococcus (GBS) bacteremia had LP-positive meningitis [47].
On the other hand, the prevalence of neonatal meningitis may vary in countries with lower levels of development as shown in the Kaul et al. [48]. study, conducted in India. This 1-year prospective study documented that 22.5% of 102 patients evaluated for late sepsis had concomitant meningitis [48].
Regarding the usefulness that urine culture may have as part of the sepsis protocol, a retrospective study of 349 neonates evaluated for early sepsis (in whom blood and urine cultures were taken), found 0% positivity in urine cultures. In the same study, a study group B with late sepsis (>6 days) was included. 25% had positive urine culture and 41.7% positive blood culture. It was also found that 38% of neonates with a positive urine culture had a positive blood culture, and 62% had a negative blood culture [49].
Another retrospective study included 1402 neonates (664 episodes of sepsis). Half of the patients underwent urine culture as part of the approach. Only in 24/212 of them (11%) with a diagnosis of sepsis was a positive urine culture identified [50].
Consensus Recommendations,
Based on the review and analysis carried out, this Consensus recommends that:
Despite the low rate of recovery, blood culture is still the gold standard for the diagnosis of neonatal sepsis, so it should continue to be taken in neonates with suspected early and late sepsis. It is recommended to take two peripheral blood cultures from 2 different puncture sites. If this is not possible, a blood culture with at least 1 mL must be taken. Level of evidence I, Degree of recommendation A
If this amount is not reached at the time of collection, but this sample is greater than 3 mL and will be processed in automated equipment, it is recommended to send the sample to be processed, under the premise that the recovery percentage may drop. Level of evidence I, Degree of recommendation B
Lumbar puncture is not recommended in all asymptomatic neonates with risk factors for sepsis. Level of evidence e II-2, Degree of recommendation A
Similarly, it is not recommended to routinely perform a lumbar puncture in all neonates with early sepsis. Level of evidence II-2, Degree of recommendation A
Lumbar puncture is certainly recommended in neonates with late sepsis, especially those with positive blood cultures. Level of evidence II-2, Degree of recommendation A
Routine urine culture is not recommended in all neonates with early sepsis. Level of evidence II-2 Degree of recommendation B
It is recommended to include urine culture in the approach of neonates with late sepsis in whom no obvious focus is identified Level of evidence II-2 Degree of recommendation A
Question 4. Based on the available evidence, how to decide the treatment scheme in cases of early neonatal sepsis, and how long should this therapy last?
One of the most repeated recommendations worldwide in the treatment of neonatal sepsis is the use of the antibiotic scheme of ampicillin plus an aminoglycoside (gentamicin or amikacin). This is based on reports from the US & other developed countries references, where Streptococcus agalactiae is the main pathogen associated with this presentation. Nonetheless, in developing countries, the isolated bacteria tend to be enterobacteria such as Escherichia coli and Klebsiella spp.
A chronological analysis of some of the studies with the largest number of confirmed sepsis cases reported in Mexico shows that the etiological agent profiles of early sepsis and late sepsis can be mixed. In the fours studies from Arredondo et al. [51], carried out in the period between the 1990s to 2020 at Civil Hospital of Guadalajara “Dr. Juan I. Menchaca”, 72% of the bacteria identified were Gram-negative bacilli; from these, Escherichia coli and Klebsiella pneumoniae were the most frequent. From the Gram eve lofes: coagulase-negative staphylococci [51-54].
The 10th Clinical Consensus, from the Ibero-American Society of Neonatology (Suspected Neonatal Sepsis) [24], presents serious reflections on the abuse of antibiotics in these neonates, with suspected maternal risk factors where the risk of early neonatal sepsis is only 0.21 ‰ in asymptomatic newborns (despite these factors), 2.6 ‰ when the performed test is questionable, and only about 11 ‰ when there is a clinical disease in the study. Thus, many newborns are treated as infected, when in fact, they are not. This consensus indicates that, depending on each country in Latin America, between 65%-95% of neonates admitted to the neonatal intensive care unit (NICU) will receive empirical antibiotics for suspected early neonatal sepsis, but only 1% to 5% of them will have positive blood cultures.
In accordance with other references, SIBEN recommends the use of ampicillin plus aminoglycoside regimen as the first alternative in suspected cases. However, as this was a consensus of “suspected sepsis” the greatest weight of its recommendation falls on the policies of, when not to start this antibiotic regimen, or when to stop them.
This SIBEN consensus, recommended that, according to current evidence, third-generation cephalosporins should not be started in suspected early sepsis, because their indiscriminate use has been associated with the rapid development of resistance and increased risk of systemic candidiasis, as well as severe complications such as necrotizing enterocolitis and death before discharge, compared with the use of ampicillin + aminoglycoside [24].
Nonetheless, a special category should be considered for premature newborns treated in second- and third-level NICUs, where systemic invasion with rupture of biological barriers can be a constant due to the need to perform assisted resuscitation briefly after birth, the insertion of endotracheal tubes, the assisted mechanical ventilation, as well as the insertion of catheters for intravenous therapy. These are the main risk factors for the development of hospital microbiota infections. Similarly, these aforementioned factors can be intermingled with the latter, further complicating the response to a management with a rigid and inflexible scheme such as ampicillin plus aminoglycoside, due to its low coverage of nosocomial microorganisms. In fact, Reyna and collaborators showed in a Perinatal Care Institute that the efficacy of this traditional scheme of ampicillin plus aminoglycoside in cases of suspected early sepsis had a failure close to 70% [55].
Consensus Recommendations.
• Respiratory Distress Syndrome (RDS), premature rupture of membranes, and suspicion of maternal chorioamnionitis, as well as the clinical visual appreciation of deterioration as isolated or combined risk factors, should not be an indication for the use of empiric antibiotic therapy. Level of evidence I, II-1, II-2, III Degree of recommendation A
• In places where Group B Streptococcus (GBS), Escherichia coli, or Klebsiella sp. Susceptible to aminoglycosides are the main agents responsible for sepsis, the combination of ampicillin and an aminoglycoside (gentamicin o amikacin) is a current recommendation to be used as initial therapy. Level of evidence I Degree of recommendation A
• If the result of the blood culture is positive, the antibiotic should be adjusted to the result of the recovered microorganism and the duration of treatment will be of 7-10 days, depending on the response to antibiotic therapy. Level of evidence I Degree of recommendation A
• In asymptomatic patients with sepsis ruled out by negative blood cultures, and with a negative laboratory profile for sepsis, the empiric antibiotic regimen should be withdrawn as soon as possible. Level of evidence II-2 Degree of recommendation A
• It is important to emphasize that, if the cultures are negative for Gram-positive cocci, vancomycin should be withdrawn and not maintained in combination with the specific antibiotic, since this pressure on the indigenous microbiota can generate the appearance of resistant strains, starting with Enterococcus and the Staphylococcu groups. The procedure must be realized similarly with Gram-positives, by withdrawing Gramnegative antibiotic regimen. Reducing the antimicrobial spectrum and/or the number of antibiotics favors a reduction in “antibiotic pressure”, a reduction in bacterial resistance, and a reduction in adverse effects and costs. Level of evidence I-2, II y III. Degree of recommendation A
• The use of aminoglycosides such as amikacin or gentamicin will be limited to their use as synergy antibiotics and their use should not exceed five days. Their use as a monotherapy in neonatal sepsis should be avoided. Level of evidence II-2 Degree of recommendation A
• Third-generation cefotaxime-type cephalosporins might seem like a reasonable alternative to an aminoglycoside. However, their effectiveness against Listeria monocytogenes and Enterococcus is poor. In addition, the rapid development of bacterial resistance of the local microbiota has been reported when cefotaxime has been used routinely for the treatment of early neonatal sepsis, so its use should be restricted to cases of suspected meningitis or with recovered susceptible microorganisms. Its greatest risk is the induction of extended-spectrum beta-lactamases (ESBLs) in Gram-negative bacteria or being a risk factor for the development of invasive candidiasis. Level of evidence I, II2, III. Degree of recommendation A
Question 5. How must the treatment scheme be decided in cases of late neonatal sepsis and how long should this therapy last?
Late neonatal sepsis is one that occurs after 72 hours after birth and is more frequent in low-birth-weight newborns who remain hospitalized in a neonatal ICU for prolonged periods.
The most important risk factors for late neonatal sepsis are the same: prematurity, the use of catheters for prolonged periods, invasive procedures, endotracheal intubation, use of H2 blockers, antibiotics of empirical therapy for more than five days, among others [56,57].
The etiology of late neonatal sepsis can vary according to each health center, despite the comparison between various studies. Nonetheless, the most repeated pathogens between the studies are: Klebsiella pneumoniae>, Enterobacter spp>, coagulase-negative Staphylococcus>, methicillin-resistant Staphylococcus aureus>, and Pseudomonas aeruginosa> [58-60].
The largest series in Mexico differ very little from this etiological pattern, with small differences in the prevalence of some of these pathogens [51-54].
The main pathogens recovered in late sepsis cases from the hospitals within the collaborative group do not differ in the etiological behavior from those reported in other studies. Among these pathogens, multidrug-resistant Klebsiella pneumoniae, Enterobacter spp, coagulase-negative Staphylococcus, methicillinresistant Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp are the main challenges of the last years.
Regarding the time of use of antibiotic regimens in confirmed neonatal sepsis in the last two decades, the ideal times for antibiotic management in confirmed sepsis in newborns have come into controversy, since the prolonged use of broad-spectrum antibiotics seems to have more risk than benefits. In addition, there are very few evidence-based guidelines that support the best useful times.
For many years, the recommended time for these schemes has had a dogmatic component, associated with level III and IV evidence (non-academic clinical consensus, expert opinions, recommendations manuals) with inflexible schemes such as 14 or 21 days.
Controlled noninferiority clinical trials such as those conducted by Reddy, and Geeta, Gathwala, and Fursule, showed that even in Gram-negative neonatal systemic infections, a 10-day schedule showed non-inferiority to a 14-day schedule, with the benefits of shorter hospital length-of-stay and less risk of antibiotic pressure that can generate dysbiosis in the patient [61-63].
On the other hand, another controlled clinical trial conducted by Chowdhary showed that a 7-day schedule has a higher risk of failure than a 10-day schedule of antibiotics in substantiated neonatal sepsis [64].
Consensus Recommendations.
Based on the fact that coagulase-negative Staphylococcus>, Staphylococcus aureus>, and Enterococcus> are the main Grampositive strains recovered in Mexico and Latin America, and that the main Gram-negatives are: Klebsiella pneumoniae>, Klebsiellas/ Enterobacter>, and Escherichia coli, this consensus recommends that the Cefotaxime + vancomycin or piperacillin + tazobactam regimens are justified as empirical therapies in neonates with multiple risk factors for infection in suspected late sepsis (more than 72 hours of hospital length-of-stay in the hospital area),Level of evidence II-2, III Degree of recommendation A
Before the isolation of pathogens such as Pseudomonas aeruginosa>, Acinetobacter spp. Stenotrophomonas maltophilia, Comamonas spp, or other microorganisms identified as multidrug resistant, the advice of specialists in infectious disease or microbiology must be sought.
The recommended time for treatment of early or late sepsis of known susceptible microorganisms is 10 days. Level of evidence I Degree of recommendation A.
Short regimens of five to seven days are not recommended in late sepsis. Level of evidence I Degree of recommendation A
Question 6. What is the recommended antibiotic regimen for the management of neonatal meningitis, and for how long should the treatment be maintained?
Neonatal bacterial meningitis continues to be a major cause of mortality and morbidity. Mortality rates attributable to neonatal meningitis remain very significant (5% a 25%) [65].
Approximately 25% to 50% of survivors experience neurological sequelae, including impaired cognitive impairment and developmental delay [66].
Like sepsis, this entity is classified as early or late onset. Earlyonset infections occur within the first 72 hours of life. Late-onset infections are predominantly observed in premature infants, with a wide variety of pathogens involved.
In the US, the incidence of early-onset meningitis has been greatly reduced by the initiation of intrapartum antibiotics to combat GBS infections. However, this pathogen remains the most common cause of both neonatal meningitis and sepsis, causing more than 40% of all early-onset infections. Though, in countries like Canada, GBS has been displaced by Escherichia coli as the main causal agent of this disease [62]. In Mexico and other Latin American countries, the most common pathogen in this group is Escherichia coli, and it remains the most frequent pathogen of sepsis and early-onset meningitis among very low birth weight infants (less than 1500 grams) [68,69].
Another strain that is sporadically found in our country in early-onset meningitis is Listeria monocytogenes [70,71]. Yet, in other Latin American countries such as Peru and Colombia, its increase has led it to occupy second place after Escherichia coli [72,73]. Therefore, antibiotic coverage in these countries should continue to consider this possibility.
Consensus Recommendations.
Neonatal meningitis constitutes a health emergency, and as soon as it is suspected, empirical antibiotic treatment should be indicated. A large part of the reviews and consensuses maintain the ampicillin plus an aminoglycoside regimen as the first management alternative in early-onset meningitis, based on the involvement of GBS, Escherichia coli, and Listeria monocytogenes. However, due to the difficulty in achieving adequate concentrations in the tissue of the central nervous system, the use of aminoglycosides is increasingly questioned. Level of evidence II-2, Degree of recommendation A
The late-onset presentation should cover organisms from the nosocomial setting, especially those in neonatal intensive care units, including multidrug-resistant Klebsiella, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus. Level of evidence II-2, II-3 and III, Degree of recommendation A
Since the eighth edition of the book “Infectious Diseases of the Fetus and Newborn Infant” by Remington and Klein [73], it is recommended that the empirical use of cefotaxime or meropenem in neonates should be restricted to cases of meningitis or isolation of Gram-negative bacteria. This situation has given rise to a shift in management recommendations, especially in countries where Gram-negatives are predominantly the cause of sepsis and meningitis. Level of evidence III, Degree of recommendation A
In cases of suspected late-onset nosocomial meningitis, where the participation of methicillin-resistant Staphylococcus aureus, as well as negative-coagulase Staphylococcus and the Enterococcus genus, in addition to Gram-negatives such as Klebsiella / Enterobacter spp, and Pseudomonas, among other pathogens, the regimen of vancomycin plus cefotaxime or meropenem is recommended as an adequate alternative. Level of evidence I, II- 2, III. Degree of recommendation A
A significant number of other rare agents reported each year worldwide as causative agents of meningitis in newborns, including Salmonella spp, Elizabethkingia meningoseptica, Serratia marcescens, among others [74-77], should be analyzed individually, and if possible, with the advice from an expert in infectious diseases or microbiology to determine treatment. The latter must be adjusted based on the same reported sensitivity profile or on the analysis of the published international experience. Level of evidence II-2, III. Degree of recommendation A
Antibiotics must be administered as soon as possible. Prior to starting antibiotics, blood cultures and lumbar punctures should be performed for both culture and cytochemical study, with the intention of having elements to corroborate the diagnosis or adjust the antimicrobial regimen specifically to the isolated microorganism.
Regarding the time that antimicrobials should be mantained, the various studies agree that the management time for Gramnegative meningeal infections should be maintained for a minimum period of three weeks. Despite the latter, a retrospective review study in China showed that the use of third-generation cephalosporins with an average of 19 days, only had a success of 77.1%. Therefore, each case must be studied individually, and both microbiological and clinical responses must be evaluated before withdrawing the treatment [78]. In the case of some Gram-positive strains such as Streptococcus, the schemes can be shortened to 14 days. There is evidence that schemes shorter than these present a greater risk of complications or relapses. Level of evidence III Degree of recommendation A
Question 7. What is the recommended antibiotic treatment for cases of bacterial neonatal pneumonia?
Neonatal pneumonia is an infection that affects the lung parenchyma of the newborn and is associated with significant morbidity and mortality. Its incidence is variable, depending on the conditions of each institution and country [79].>
Its presentation can be congenital, as well as identified as early or late. Congenital or early-onset pneumonia usually presents within the first 72 hours of life, and is acquired from the mother through the following mechanisms:
• Intrauterine aspiration of infected amniotic fluid, transplacental transmission from the maternal circulation.
• Aspiration during or immediately after delivery of amniotic fluid and/or infected vaginal secretions.
• Organisms present in vaginal secretions can colonize the newborn and, depending on the conditions of the newborn, invade the lower respiratory tract and cause pneumonia.
Late-onset pneumonia presents after 3 days of life and can occur during hospitalization or after hospital discharge. It is generally caused by microorganisms present in the hospital environment (nosocomial) and transmitted by infected newborns, infected personnel, or contaminated ventilation equipment. Microorganisms can enter through solutions of the rupture of the defense mechanisms in the tracheal or bronchial mucosa, the use of assisted ventilation devices, or through the bloodstream [80].
Its clinical presentation generally presents with data of earlyonset respiratory distress, which can be associated with lethargy, apnea, tachycardia, dysthermia, and poor tissue perfusion. In late-onset pneumonia, the clinic is characterized by deterioration of the newborn’s baseline condition, which includes nonspecific signs such as apnea, tachypnea, anorexia, abdominal distension, jaundice, vomiting, respiratory distress, and signs of shock.
Patients who are ventilated may present a deterioration in their respiratory condition with an increase in oxygen requirements and ventilatory parameters, associated with mucopurulent secretions in bronchial aspiration [79-82].
The etiology may depend on various factors such as maternal colonization, the season (community pneumonia), as well as the hospital microbiota.
In countries such as the US, the main causal agent of early pneumonia continues to be Streptococcus agalactiae, based on the high levels of maternal vaginal colonization by this pathogen. In Mexico, enterobacteria such as Escherichia coli and Klebsiella spp. Are the main pathogens responsible for early pneumonia, although some cases of infection by Streptoccocus agalatiae, Streptococcus pneumoniae, and Listeria monocytogenes, have been reported sporadically [70,71,83,84].
In cases of late-onset pneumonia, S. aureus, S. epidermidis, K. pneumoniae, P. aeruginosa, among other pathogens, should be considered as the possible etiological agents.
In neonatal pneumonia, there is a component of early infection with the late presentation, usually after the second week of birth, which includes Chlamydia trachomatis and mycoplasma infections [80].
There are few randomized clinical studies of treatment for neonatal pneumonia., Therefore, there is a need to establish an empirical antibiotic regimen in situations of clinical suspicion, without microbiological or radiological confirmation since the appearance of consolidations can be delayed until the following 72 hours of clinical onset [86].
The decision should be based on the isolation patterns and antimicrobial resistance known locally or regionally in each population or institution, associated with the clinical data presented by each patient [87].
In cases where the pathogen is detected in blood cultures or in bronchial aspirates, the antibiotic scheme should be adjusted according to the antibiogram report.
In cases of early pneumonia, the regimen is the one that covers the most common germs (ampicillin association) and an aminoglycoside (gentamicin or amikacin) as the first choice.
In late infections of nosocomial origin, the ideal is to prescribe a combination of antibiotics for which the most frequent germs of each service are sensitive. In these cases, the use of cefotaxime plus vancomycin may be considered, mainly for coverage against methicillin-resistant S. aureus or coagulase-negative Staphylococcus. Nonetheless, in cases in which the participation of agents with resistance to third-generation cephalosporins is suspected, the adjustment must be carried out under the supervision of an expert in infectious diseases and based on the known evidence on the microbiological profile of each unit.
In relation to the recommended time for the antibiotic management of neonatal pneumonia, these times have been managed arbitrarily most of the time, using 10 to 14 days [88].
In cases of mild community-acquired pneumonia (CAP) in newborns, a controlled clinical trial compared the effect of a 4-day course (testing group) with a 7-day course (control group) of antibiotic regimen on the treatment success rate. The infants were randomized to receive a total of 4 days of antibiotics (Group 1) or 7 days of antibiotics (Group 2). The outcome measure was treatment failure in each group within 3 days of discharge. This study reported that the treatment success rate of both groups was 100%, concluding that for infants who become clinically asymptomatic within 48 h of antibiotic therapy, the 4-day schedule of antibiotic therapy is effective and safe as 7 days, with a significant reduction in hospital length-of-stay, antibiotic use, and cost [88].
Concerning to pneumonia due to “atypical” microorganisms, a randomized placebo-controlled study found that the use of clarithromycin resulted in the eradication of Ureaplasma urealyticum [89]. Therefore, in patients treated with suspected or diagnosed infection by Ureaplasma urealyticum, other mycoplasmas, or Chlamydia trachomatis, it is recommended to start intravenous treatment with Clarithromycin at a dose of 7.5 mg/kg/dose/12 hours.
Consensus Recommendations
In cases of early pneumonia, when the recommended antibiotic regimen as the first option is ampicillin + aminoglycoside (gentamicin or amikacin, preferably in a daily dose), and when the response is adequate, its recommended duration should not exceed 7 days. Level of evidence II-3, III Degree of recommendation A
In late infections of nosocomial origin, the ideal is to prescribe a combination of antibiotics for which the most frequent microorganisms are sensitive.
Daily or routine tracheal aspirate cultures and chest radiographs are not recommended for neonates with pneumonia. The case should be individualized based on clinical symptoms and risk factors, and the results should be interpreted with caution.
Level of evidence III, Degree of recommendation D
In patients with suspected or diagnosed infection by Ureaplasma urealyticum, or Chlamydia trachomatis, it is recommended to start intravenous treatment with Clarithromycin. Level of evidence I. Degree of recommendation B.
Question 8. What is the recommended antibiotic treatment for necrotizing enterocolitis in newborns, and what is the ideal time of treatment?
Between 2 to 5% of all infants admitted to the NICU develop necrotizing enterocolitis (NEC), with 90% of them being premature. This disease occurs in 2% to 8% of very low birth weight (VLBW) infants.
Approximately 20% to 40% of these neonates may require surgical intervention. The mortality rate varies from 20% to 35%, and 25% of survivors experience long-term sequelae, such as poor growth, bowel syndrome, and intestinal stricture [90].
The most widely used definition in the field is through the modified Bell scale. Nonetheless, it is not a definition, but a staging based on an already made diagnosis; with the problem being that the criteria include signs and symptoms with variations in sensitivity and specificity that are not consistent with the importance of the diagnosis (e.g. feeding intolerance and blood in stools). The latter causes the over or underestimation of the disease [91,92].
In VLBW newborns, Bell scale grade I symptoms such as oral intolerance and abdominal distention are common normal findings, or they are part of other conditions such as sepsis. For this reason and purposes of this consensus, we recommend the use of antibiotics in NEC using the modified Bell scale, from stage ≥ II [93].
The clinical manifestations associated with NEC are food intolerance, abdominal distension, and bloody stools after 8 to 10 days of age. The first imaging signs that should lead to suspicion of necrotizing enterocolitis include dilated intestinal loops and gas shortage. Pathogmonomic findings on abdominal radiography are pneumatosis intestinalis, portal venous gas, or both.
In diagnostic aids, thrombocytopenia stands out with a rapid decrease as a sign of progression to a poor prognosis, alterations in the blood count (leukopenia or leukocytosis), elevation fo acute phase reactants C-reactive protein and procalcitonin, as well as peripheral blood cultures that can be positive in 30% to 35%. Furthermore, glycemic changes, hyponatremia, alterations in coagulation times (PT and PTT), and arterial blood gases (metabolic acidosis) can be added [94].
The infectious etiology of NEC usually have a polymicrobial component, where the microorganisms involved are the microbiota found in the large intestine, including Escherichiacoli, Klebsiella/ Enterobacter, Citrobacter, Serratia, Acinetobacter, Pseudomonas, Salmonella, coagulase-negative Staphylococcus and Staphylococcus aureus. Moreover, anaerobes such as Clostridia, Bacteroides, some mycoplasmas and viruses have also been described for this pathology, as well as the participation of fungi [95].
Medical management includes medical care with empiric antibiotic therapy to prevent disease progression and surgical intervention. However, there is no evidence-based consensus regarding empiric therapy or timing surgery. In a systematic review, no antibiotic regimen was found to be superior to ampicillin and gentamicin in decreasing mortality or preventing clinical deterioration, among 3,161 patients from 2 controlled and 3 observational studies, in which 3 studies evaluated the addition of anaerobic coverage, and one observational study included the comparison with cefotaxime and vancomycin [96].
In another Cochrane systematic review, where the efficacy of different antibiotic schemes on mortality, and the need for surgery in neonates with NEC was compared, selecting all randomized and quasi-randomized clinical trials where antibiotics were used for the treatment of NEC. We found that, in one of the trials, 42 preterm infants received IV ampicillin & gentamicin or ampicillin, gentamicin and clindamycin. The results found no statistically significant difference in mortality (RR 1.10; CI 95%, 0.32 to 3.83), or bowel perforation (RR 2.20; CI 95% 0.45 to 10.74) between both groups, but there was a greater tendency towards stenosis in the group that received clindamycin. In another trial, 20 neonates received intravenous ampicillin and gentamicin with or without gentamicin, with no significant difference in mortality, intestinal perforation, or development of stenosis [97].
The empiric antibiotic regimen should provide coverage for the common pathogens of late-onset neonatal sepsis. In addition, in events of peritonitis or pneumoperitoneum suggestive of intestinal perforation, coverage should be provided to anaerobes.
Consensus Recommendations
The recommended treatment guidelines are as follows:
• Stage I Necrotizing Enterocolitis: Do not administer antibiotic treatment.
• Stage II A-B Necrotizing Enterocolitis: A-B coverage for gram-negative and positive bacteria (Ampicillin and gentamicin for 7 days).4 Level of evidence II-1 Degree of recommendation A
• Stage III A-B Necrotizing Enterocolitis or proven perforation: Add a third antibiotic with anaerobic coverage. Level of evidence II-1 Degree of recommendation A
• Option 1: Ampicillin, gentamicin, and metronidazole for 7 to 10 days or 3 to 5 days after surgical resolution. Level of evidence II-1
• Option 2: Piperacillin/Tazobactam monotherapy; consider in patients without evidence of shock. Level of evidence II-1
• Limit the use of carbapenems to situations of: necrotizing enterocolitis with septic shock in patients with extended-spectrum beta-lactamase-(ESBL) producing Enterobacteriaceae. Level of evidence I Degree of recommendation A
• In those cases, in which the clinical evolution is favorable, the blood culture is negative, the tests are of low infectious risk, and the symptoms can be attributed to other non-infectious causes: the antibiotics will be withdrawn after 48-72 hours.
• When microbiological isolation is achieved by cultures, treatment should be directed according to the sensitivity profile and, where appropriate and possible, de-escalate the antibiotic. Level of evidence I Degree of recommendation A
Question 9 What antifungal must be recommended, based on the best evidence, for the empirical treatment of patients suspected of invasive fungal infections in the neonatal stage?
Invasive fungal infections in NICUs are the second leading cause of infection-related mortality in critically ill newborns, especially in premature infants, and those with very low birth weight (<1500 g), presenting also a health problem due to the associated morbidity. Nearly 50% of surviving neonates will have serious long-term neurodevelopmental deficits, including cerebral palsy, hearing or cognitive impairment, eye damage, or periventricular leukomalacia; especially among the lowest gestational age and lowest weight at birth [98-100].
Neonatal invasive fungal infection is largely synonymous with invasive Candida infection; however, cases due to other fungi such as Malassezia, Aspergillus and Zygomycetes are being increasingly reported [98-100].
Invasive candidiasis is the third most common cause of late-onset sepsis in patients with very low birth weight, and a significant cause of morbidity and mortality in NICU. Its incidence varies from 0.5% to 20% and is inversely correlated with birth weight [101].
The most common species of neonatal candidemia is Candida albicans, being isolated in 60 to 75% of infections, followed by Candida parapsilosis, with 14 to 30% of isolates. Other species like C. tropicalis, C. lusitaniae, C. glabratay C. kruseison are much less frequent [98-101].
The main risk factors for the development of neonatal invasive fungal infections are those linked to premature birth and low weight, such as: gestational age <28 weeks, and weight <1500g, the use of parenteral nutrition, prolonged use of a central venous catheter, mechanical ventilation, prolonged length-of-stay in the NICU, use of corticosteroids, delay in enteral feeding, and biological factors such as the presence of persistent neutropenia, hyperglycemia, and thrombocytopenia, but are particularly associated with the use of broad-spectrum antibiotics in multiple schemes or prolonged periods [102-104].
The clinical features of invasive candidiasis in the newborn are nonspecific, indistinguishable from those caused by late-onset neonatal sepsis. They may include lethargy, apnea, respiratory distress, cardiovascular instability and/or feeding intolerance. It can present as candidemia, urinary tract infection, and/or as the compromise of any other tissue. A syndrome particularly unique to preterm infants is Hematogenous Candida Meningoencephalitis (HCME). Up to 25% of invasive candidiasis in premature newborns are associated with meningoencephalitis, even if they do not present obvious neurological symptoms. Although the latter condition is the most frequent, central nervous system diseases caused by Candida can also present in the form of brain abscesses, ventriculitis, and vasculitis [103,104].
PERSISTENT CANDIDEMIA AND NEURODEVELOPMENTAL IMPAIRMENT
Neonates frequently present with persistent candidemia (positive blood cultures for more than 72 hours in a patient receiving specific therapy). Candidemia, especially when present for more than 5 days, can spread to multiple organs such as kidneys (5-30%), heart (5-15%), eyes (3-50%), bones, joints, skin, and lungs. What favors the appearance of serious complications is the dissemination to different organs and systems. When the central nervous system is affected, studies have shown that up to 73% of low birth weight infants die or show signs of impaired neurodevelopment up to 18 to 22 months of follow-up [105-107].
The proper use of antifungals in the neonatal population is important for both the prevention and treatment of Invasive Fungal Infection (IFI). To maximize the activity of antifungal agents and minimize toxicity in this vulnerable population, the isolation, location, hemodynamic, stability, and extension of the infection should be assessed when choosing treatment. Because central nervous system involvement is common in newborns, a drug that crosses the blood-brain barrier and with fungicidal activity must be selected. It is also important to know if there is a urinary tract infection, which is a frequent situation in newborns and that will condition the optimal use of a drug with good diffusion in urine [108,109].
]Studies of the extension of the infection are recommended: lumbar puncture, eye fundus examination, echocardiography, and abdominal ultrasound in all cases of candidemia and also renal ultrasound in the case of candiduria. Replacement of the intravascular catheter is also recommended [106,109,110].
Antifungals that have been used for the management of invasive fungal infections in newborns are grouped into four categories [110] (Table 4). Unlike in adults and non-neonatal pediatric patients, newborns tolerate these quite well, including drugs such as Amphotericin-B Deoxycholate, without significant nephrotoxic effects. On the other hand, drugs such as echinocandins do not easily reach therapeutic concentrations, mainly in the urinary tract and the central nervous system [98,109,111].
In any premature neonate with clinical suspicion or microbiological evidence of invasive candidiasis, a disseminated disease should be assumed and therefore a thorough clinical examination should be carried out. Particularly, it is of the utmost importance to consider the possibility of HCME, so in antifungal therapy, central nervous system coverage should be considered [112].
The epidemiology files of the Maternal and Perinatal Hospital “Mónica Pretelini Sáenz” and the Children’s Hospital of the State of Mexico show an increase in the number of cases of fungal infection in the neonatal period in the last two years (Tables 4 & 5)
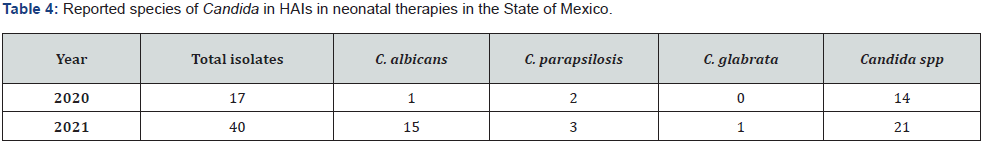
Source: Epidemiology files of the Perinatal Hospital “Mónica Pretelini Sáenz” and the Children’s Hospital of the State of Mexico.
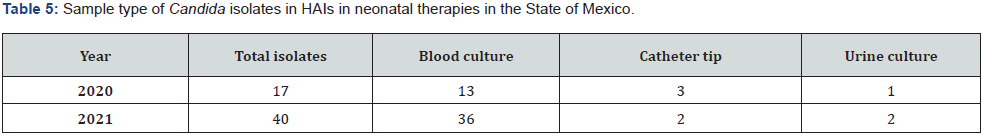
Source: Epidemiology files of the Perinatal Hospital “Mónica Pretelini Sáenz” and the Children’s Hospital of the State of Mexico.
Consensus Recommendations
Most of the revised guidelines suggest the use of Amphotericin B-Deoxycholate as first-line and two drugs as second-line with their respective considerations: Liposomal Amphotericin B (in the presence of urinary tract involvement it is not recommended) and Fluconazole which is recommended in patients in whom azole prophylaxis has not been administered, and not first-line if the patient has hemodynamic instability [106,109,111]. Level of Evidence I Degree of Recommendation A
Echinocandins are increasingly used in these patients. Nonetheless, they should be used with caution and generally as rescue therapy. Especially in situations where the use of Amphotericin B Deoxycholate or Fluconazole is not possible due to toxicity or resistance. Level of Evidence II-3 Degree of Recommendation B
Micafungin, although it is a drug recommended for the treatment of neonatal IC, its penetration into the central nervous system is known to be dose-dependent. Thus, high doses of 10mg/ kg/day should be used if it is decided to use in suspected HCME [111,112]. Level of Evidence I, II-3 Degree of Recommendation A
Prophylaxis of Candida infection in newborns.
Not all newborns need prophylaxis for invasive candidiasis. As mentioned above, it is known that different risk factors impact the incidence of this entity in NICU. According to revised guidelines [105,106,112-114] (Table 6) the recommendations are as follows:
1. Newborns weighing <1000 g. Fluconazole prophylaxis.
2. Newborns weighing <1500 g. Nystatin prophylaxis, as an alternative when fluconazole is not available. (Consider the potential risk of developing Necrotizing Enterocolitis)
3. Fluconazole prophylaxis when the incidence of invasive Candidiasis in NICU is ≥5%
4. European guidelines recommend, the use of lactoferrin 100mg/day ± Lactobacillus rhamnosus, a dose of 106 CFU/day from the third day of life until the end of the sixth week of life or until discharge from the NICU in newborns weighing <1500 g.
5. Miconazole is contraindicated since its use may favor the appearance of azole resistance. Level of Evidence I Degree of Recommendation A
Question 10. Is it advisable to implement pharmacovigilance programs in newborns treated with antibiotics?
During intrauterine life, the fetus can be exposed to a series of drugs and toxic substances whose effects can be immediate and cause fetal death or cause damage that can manifest at birth or even weeks, months, or years later [3,115-117]. (Level of Evidence III Degree of Recommendation B)
55-65% of pregnant women and more than 70% of newborns admitted to NICU receive antibiotics during their hospitalizations [3,117,118]. (Level of Evidence III Degree of Recommendation B)
Pharmacovigilance is the science and activities related to the detection, evaluation, understanding, and prevention of Adverse Drug Reactions (ADRs) or any other potential drug-related problem.
The specific objectives of pharmacovigilance are:
• Improve patient care and safety in relation to the use of medications and all medical interventions.
• Improve public health and safety in relation to the use of medicines.
• Contribute to the evaluation of the benefits, harms, efficacy, and risk of medicines, as well as promoting their safe, rational, and more efficient (and even more profitable) use.
The success or failure of any spontaneous notification system depends on participation. Health professionals have been the main providers of notifications of suspected ADR cases throughout the history of pharmacovigilance.
Wherever medicines are used, they must be willing to observe and report unwanted adverse events [119].
To achieve something closer to ideal practices, more attention should be paid to training health professionals in the diagnosis, management, and prevention of ADRs. Not all signs are so specific or dramatic to be easily diagnosed, as were phocomelia and micromyelia caused by thalidomide [120].
The implementation of a pharmacovigilance program must be carried out in accordance with the current applicable regulations, such as the Official Mexican Normative NOM-220-SSA1-2016, Installation and operation of pharmacovigilance [121]. The latter serves as a great reference to achieve the correct implementation of a pharmacovigilance program. Since it establishes the guidelines for the installation and operation of pharmacovigilance in the national territory, it highlights everything related to: the types of notification criteria, criteria to determine the degree of information, criteria to determine the seriousness of a case, criteria to determine the severity of a case, the classification of reactions, the sending times of notifications, the reception channels, the notification forms, and the requirements for the receipt of notifications [122,123].
To carry out pharmacovigilance, some points are listed that will be informative but not limited to:
Identification of signs suggesting ADRs
• Performs the required investigation and collects the necessary information to fill out the report format for the Notice of Suspected Adverse Drug Reactions issued by COFEPRIS, by reviewing the clinical file, interviewing the doctor, the nurse, or the patient himself.
• Verifies the consistency of the ADR suspicion data, taking into account the evaluation of the causality of said event.
• Fills out the Notice of Suspected Adverse Drug Reactions forms, in accordance with the current COFEPRIS guidelines.
• Classifies ADR based on severity and evaluates its causality.
• Reviews the evolution of the patient after presenting the clinical manifestation of ADR.
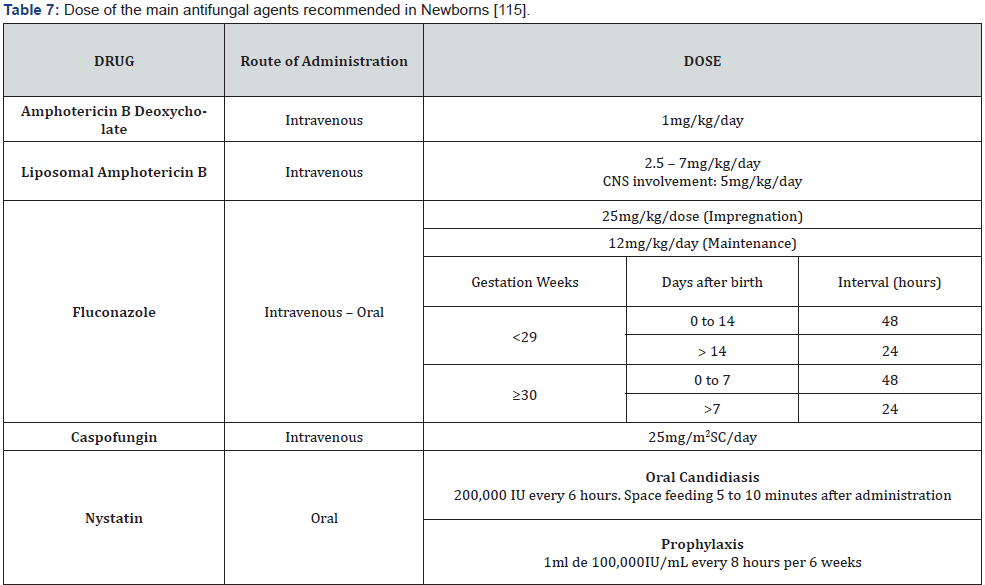
• Carry out the ADR notification to the corresponding authority (Table 7) [115].
Conclusion
Based on the recommendations of this consensus, this collaborative group concluded the need to establish policies of prudent and standardized use under the best evidence regarding the of antibiotics in NICUs of the hospitals of the Institute of Health of the State of Mexico. Under these guidelines, the integration of a common manual for the use of antibiotics and antifungals must be reviewed periodically, in addition to strengthening antibiotic pharmacovigilance programs in the NICUs.
Declaration of Conflict of Interest
The authors declare that there is no known competing financial interest or personal relationships that could have appeared to influence the work reported in this consensus.
References
- Sutherland JM, Keller WH (1961) Novobiocin and Neonatal Hyperbilirubinemia: An Investigation of the Relationship in an Epidemic of Neonatal Hyperbilirubinemia. Am J Dis Child 101(4): 447-453.
- Sutherland JM (1959) Fatal cardiovascular collapse of infants receiving large amounts of chloramphenicol. AMA journal diseases of children 97(6): 761-767.
- Cardetti M, Rodríguez S, Sola A (2020) Uso (y abuso) de antibióticos en la medicina perinatal. Anales de Pediatría 93(3): 207.e1-207.e7.
- Ferrario C, Califano G, Durán P, Maccarone M, Miceli I, et al. (2012) Lineamientos para la elaboración de consensos. Arch Argent Pediatr 110(2): 163-167.
- The AGREE Collaboration Appraisal of Guidelines for Research & Evaluation (AGREE) Instrument.
- Birtwhistle R, Pottie K, Shaw E, Dickinson JA, Brauer P, et al. (2012) Canadian task forcé on preventive health care: Can Fam Physician 58 (1): 13-15.
- Manterola C, Asenjo-Lobos C, Otzen T (2014) Jerarquización de la evidencia. Niveles de evidencia y grados de recomendación de uso actual. Rev Chilena Infectol 31(6): 705-718.
- Wynn J, Wong H, Shanley TP, Bizzarro MJ, Saiman L, et al. (2014) Time for a neonatal consensus definition of sepsis. Pediatr Crit Med Care 15(6): 523-528.
- McGovern M, Giannoni E, Kuester H, Turner MA, Hoogen A, et al. (2020) Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res 88(1): 14-26.
- Wynn J (2016) Defining Neonatal Sepsis. Curr Opin Pediatric 28(2): 135-140.
- Hofer N, Müller W, Resch B (2012) Definition of SIRS and sepsis in correlation with early and late onset neonatal sepsis J Pediatr Intensive Care 1(1): 17-23.
- Glaser MA, Hughes LM, Jnah A, Newberry D (2020) Neonatal sepsis. Adv Neonatal Care 21(1): 49-60.
- Shane, Sanchez P, Stoll B (2017) Neonatal sepsis. Lancet 390(10104): 1770-1780.
- Reyna-Figueroa J, Yuri-Toala E, Ortiz-Ibarra FJ, Rodríguez-Ramírez E, Limón-Rojas AE (2006) Disparidad en los criterios para incluir pacientes con sepsis neonatal en estudios médicos científicos. ¿Nadamos en un mar sin límites? Anales de Pediatría; 65(6): 536-540.
- Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6(1): 2–8.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8): 801–810.
- Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, et al. (2012) Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 88(Suppl 2): S69–S74.
- Hofer N, Zacharias E, Muller W, Resch B (2012) Performance of the definitions of the systemic inflammatory response syndrome and sepsis in neonates. J Perinat Med 40(5): 587–590.
- Mohsen AH, Kamel BA (2015) Predictive values for procalcitonin in the diagnosis of neonatal sepsis. Electron Physician 7(4): 1190-1195.
- Park IH, Lee SH, Yu ST, Oh YK (2014) Serum procalcitonin as a diagnostic marker of neonatal sepsis. Korean J Pediatr 57(10): 451-456.
- Eschborn S, Weitkamp JH (2019) Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol 39(7): 893-903.
- Bunduki GK, Adu-Sarkodie Y (2020) The usefulness of C-reactive protein as a biomarker in predicting neonatal sepsis in a sub-Saharan African region. BMC Res Notes 13(1): 194.
- Xu L, Li Q, Mo Z, You P (2016) Diagnostic value of C-reactive protein in neonatal sepsis: A meta-analysis. European Journal of Inflammation 14(2): 100-108.
- Sola A, Mir R, Lemus L, Fariña D, Ortiz J, et al. (2020) Suspected Neonatal Sepsis: Tenth Clinical Consensus of the Ibero-American Society of Neonatology (SIBEN). Neoreviews 21(8): e505-e534.
- Husada, P Chanthavanich, U Chotigeat, Sunttarattiwong P, Sirivichayakul C, et. al. (2020) Predictive model for bacterial late-onset sepsis in a tertiary care hospital in Thailand. BMC Infect Dis 20(1): 151.
- Das A, Shukla S, Rahman N, Gunzler D, Abughali N (2016) Clinical indicators of Late-onset sepsis workup in very Low-Birth-Weight Infants in the neonatal intensive care unit. Am J Perinatol 33(9): 856-860.
- Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE (2010) Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza- Tanzania. BMC Pediatr 10: 39.
- Weber MW, Carlin JB, Gatchalian S, Lehmann D, Muhe L, et al. (2003) Predictors of neonatal sepsis in developing countries. Pediatr Infect Dis J 22(8): 711-717.
- Verstraete EH, Blot K, Mahieu L, Vogelaers D, Blot S (2014) Prediction models for neonatal health care-associated sepsis: a meta-analysis. Pediatrics 135(4): e1002-e1014.
- Benitz WE, Gould JB, Druzin ML (1999) Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics 103(6): e77.
- Masanja PP, Kibusi SM, Mkhoi ML (2020) Predictors of Early Onset Neonatal Sepsis among Neonates in Dodoma, Tanzania: A Case Control Study. J Trop Pediatr 66(3): 257-266.
- Fell DB, Hawken S, Wong CA, Wilson LA, Murphy MSQ, et al. (2017) Using newborn screening analytes to identify cases of neonatal sepsis. Sci Rep 7(1): 18020.
- Hershkovich O, Friedlander A, Gordon B, Arzi H, Derazne E, et al. (2013) Associations of body mass index and body height with low back pain in 829,791 adolescents. Am J Epidemiol 178(4): 603-609.
- Pettinger KJ, Mayers K, McKechnie L, Phillips B (2019) Sensitivity of the Kaiser Permanente early-onset sepsis calculator: A systematic review and meta-analysis. E Clinical Medicine 19: 100227.
- Deshmukh M, Mehta S, Patole S (2021) Sepsis calculator for neonatal early onset sepsis - a systematic review and meta-analysis. J Matern Fetal Neonatal Med 34(11): 1832-1840.
- Achten NB, Klingenberg C, Benitz WE, Stocker M, Schlapbach LJ, et. al. (2019) Association of use of the Neonatal Early-Onset Sepsis Calculator with Reduction in Antibiotic Therapy and Safety. JAMA Pediatr 173(11): 1032-1040.
- Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. (2018) Culture-Negative Early-Onset Neonatal Sepsis-At the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr 6: 285.
- Schelonka RL, M K Chai, Yoder A, Hensley D, Brockett RM, et al. (1996) Volume of blood required to detect comon neonatal pathogens. J Pediatrics 129(2): 275-278.
- Yaacobi N, Bar-Meir M, Shchors I, Bromiker R (2015) A prospective controlled trial of the optimal volume for neonatal blood cultures. Pediatr Infect Dis J 34(4): 351-354.
- Tomar P, Garg A, Gupta R, Singh A, Gupta NK, et al. (2017) Simultaneous two-site blood culture for giagnosis of Neonatal sepsis. Indian Pediatr 54(3): 199-203.
- Ortiz–Ibarra FJ, Oliva-Marín JE, Reyna-Figueroa J, Soriano-Becerril DM (2005) Evaluación controlada del volumen de sangre necesario para la detección de bacteriemia o fungemia en recién nacidos. Revista de Enfermedades Infecciosas en Pediatría 73: 1-7.
- Ortiz Ibarra J, Trevino Valdez P, Valenzuela Méndez E, Rojas LA, Flores GL, et al. (2015) Evaluation of the Light-Cycler® SeptiFast Test in Newborns With Suspicion of Nosocomial Sepsis. Iran J Pediatr 25(1): e253.
- Celik IH, Hanna M, Canpolat FE, Pammi M (2022) Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res 91(2): 337-350.
- Johnson CE, Whitwell JK, Pethe K, Saxena K, Super DM (1997) Term newborns who are at risk for sepsis: are lumbar punctures necessary? Pediatrics 99(4): E10.
- Weiss MG, Ionides SP, Anderson CL (1991) Meningitis in premature infants with respiratory distress: role of admission lumbar puncture. J Pediatr 119(6): 973–975.
- Smith PB, Garges HP, Cotton CM, Walsh TJ, Clark RH, et al. (2008) Meningitis in preterm neonates: importance of cerebrospinal fluid parameters. Am J Perinatol 25(7): 421-426.
- Ansong AK, Smith PB, Benjamin DK, Clark RH, Li JS, et al. (2009) Group B streptococcal meningitis: cerebrospinal fluid parameters in the era of intrapartum antibiotic prophylaxis. Early Hum Dev 85(10 Suppl): S5-S7.
- Kaul V, Harish R, Ganjoo S, Mahajan B, Raina SK, et al. (2013) Importance of obtaining lumbar puncture in neonates with late onset septicemia a hospital based observational study from north-west India. J Clin Neonatol 2(2): 83-87.
- Tamim MM, Alesseh H, Aziz H (2003) Analysis of the efficacy of urine culture as part of sepsis evaluation in the premature infant. Pediatr Infect Dis J 22(9): 805-808.
- Mohseny AB, van Velze V, Steggerda SJ, Smits-Wintjens VEHJ, Bekker V, et al. (2018) Late-onset sepsis due to urinary tract infection in very preterm neonates is not uncommon. Eur J Pediatr 177(1): 33-38.
- Arredondo GJL, Ortíz IFJ, Solórzano SF, Segura CE; Beltrán ZM (1994) Etiología de la septicemia neonatal en una unidad de Perinatología. Análisis de siete años. Bol Med Hosp Infant Mex 51(56): 317-322.
- Simental SP, Valenzuela FA, Avendaño BE, Plascencia IS, Martínez ND (2007) Agentes causales de sepsis neonatal temprana y tardía: una revisión de diez años en el “Hospital Infantil Privado. REVISTA DE ENFERMEDADES INFECCIOSAS EN PEDIATRÍA 20(80): 99-105.
- Pérez R, Lona JC, Quiles M, Verdugo MA, Ascencio EA, et al. (2015) Sepsis neonatal temprana, incidencia y factores de riesgo asociados en un hospital público del occidente de México. Rev Chilena Infectol 32(4): 387-392.
- Ulloa-Ricárdez A, Salazar-Espino B (2019) Epidemiología de infección neonatal temprana y tardía en una Unidad de Cuidados Intensivos Neonatales. Rev Hosp Jua Mex 86(3): 110-115.
- Reyna-Figueroa J, Ortiz-Ibarra FJ, Esteves Jaramillo A, Reyna-Figueroa J (2009) Costo económico marginal del fracaso terapéutico con ampicilina más amikacina en el tratamiento de la sepsis neonatal temprana. An Pediatr (Barc) 71(1): 54-59.
- Procianoy RS, Silveira RC (2020) The challenges of neonatal sepsis management. J Pediatr J Pediatr (Rio J) 96(Suppl 1): 80-86.
- Dong Y, Speer CP (2015) Late-Onset Neonatal Sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed100(3): 257-263.
- Mohsen L, Ramy N, Saied D, Akmal D, Salama N, et al. (2017) Emerging antimicrobial resistance in early and late-onset neonatal sepsis. Antimicrob Resist Infect Control 6: 63.
- Giannoni E, Agyeman PKA, Stocker M, Posfay-Barbe KM, Heininger U, et al. (2018) Neonatal Sepsis of Early Onset, and Hospital-Acquired and Community-Acquired Late Onset: A Prospective Population-Based Cohort Study. J Pediatr 201: 106-114.e4.
- Berlak N, Shany E, Ben-Shimol S, Chertok IA, Goldinger G, et al. (2018) Late onset sepsis: comparison between coagulase-negative staphylococci and other bacteria in the neonatal intensive care unit. Infect Dis (Lond) 50(10): 764-770.
- Reddy A, Sathenahalli V, Shivanna N, Benakappa N, Bandiya P (2021) Ten Versus 14 Days of Antibiotic Therapy in Culture-Proven Neonatal Sepsis: A Randomized, Controlled Trial. Indian J Pediatr 89(4): 339-342.
- Gathwala G, Sindwani A, Singh J, Choudhry O, Chaudhary U (2010) Ten Days vs. 14 days Antibiotic Therapy in Culture-Proven Neonatal Sepsis. J Trop Pediatr 56(6): 433-435.
- Chowdhary G, Dutta S, Narang A (2006) Randomized controlled trial of 7-Day vs. 14-Day antibiotics for neonatal sepsis. J Trop Pediatr 52(6): 427-432.
- Fursule A, Thakur A, Garg P, Kler N (2022) Duration of Antibiotic Therapy in Neonatal Gram-negative Bacterial Sepsis-10 Days Versus 14 Days. Pediatri Infect Dis J 41(2): 156-160.
- KwangSik Kim. (2015) Neonatal Bacterial Meningitis. NeoReviews 16: e535.
- Zainel A, Mitchell H, Sadarangani M (2021) Bacterial Meningitis in Children: Neurological Complications, Associated Risk Factors, and Prevention. Microorganisms 9: 535.
- Ku LC, Boggess KA, Cohen-Wolkowiez M (2015) Bacterial meningitis in infants. Clin Perinatol 42(1): 29-45.
- El-Naggar W, Afifi J, McMillan D, Toye J, Ting J, et al. (2019) Epidemiology of Meningitis in Canadian Neonatal Intensive Care Units. Pediatr Infect Dis J 38(5): 476-480.
- Reyna FJ, Ortíz IFJ, Plazola CNG, Limón RAE (2004) Meningitis bacteriana en recién nacidos. Experiencia en el Instituto Nacional de Perinatología de 1990 a 1999. Bol Med Hosp Infant Mex 61(5): 402-411.
- Solórzano SF, Arredondo GJL, Udaeta ME, Ortíz IFJ, Echaniz AG, et al. (1989) Infección sistémica neonatal por Listeria monocytogenes. Bol Med Hosp Infant Mex 46: 709-714.
- Arredondo Garcia JL, Ortiz Ibarra FJ, Sabais Herrera GA, Miguel S (2000) Recién nacido con flacidez generalizada, lesiones dérmicas diseminadas, hemorragia conjuntival bilateral, hepatomegalia e insuficiencia respiratoria. Gac Med Mex 136(6): 595-600.
- Guillén-Pinto D, Málaga-Espinoza B, Ye-Tay J, Rospigliosi-López ML, Montenegro Rivera A, et al. (2020) Meningitis neonatal: estudio multicéntrico en Lima, Perú. Rev Peru Med Exp Salud Publica 37(2): 210-219.
- Vélez Leal JL, Dávila Ramírez F (2015) Listeriosis neonatal en Colombia... ¿Igual que hace veinte años? Rev. Cienc. Salud13: 301-308.
- Victor Nizet Jerome O. Klein (2015) Bacterial sepsis and meningitis. en: Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant. (8 Edn) Elsevier Health Pp. 222-275.
- Garcia-Rodriguez E, Calderon-Lopez G, Navarro Marchena L, Pavon-Delgado A (2014) Lesiones secundarias a meningitis neonatal por Salmonella. An pediatr 81(3): 194-195.
- Chamalla SK, Karri SS, Koripadu S, Mohan N (2018) Elizabeth kingiameningoseptica: An emerging pathogen causing neonatal meningitis. J NTR Univ Health Sci 7: 213-215.
- Zaidi M, Sifuentes J, Bobadilla M, Moncada D, de León SP (1989) Epidemic of Serratia marcescens Bacteremia and Meningitis in a Neonatal Unit in Mexico City. Infect Control Hosp Epidemiol 10(1): 14-20.
- Biset S, Benti A, Molla L, Yimer S, Cherkos T, et al. (2021) Etiology of Neonatal Bacterial Meningitis and Their Antibiotic Susceptibility Pattern at the University of Gondar Comprehensive Specialized Hospital, Ethiopia: A Seven-Year Retrospective Study. Infect Drug Resist 14: 1703-1711.
- Duke T (2005) Neonatal pneumonia in developing countries. Arch Dis Child fetal Neonatal Ed 90(3): 211-219.
- Balboa de Paz F, Rueda E, Paredes MC, Gomes EB (2008) Neumonías neonatales. Acta Pediatr Esp 66(10): 481-486.
- Barnett ED, Klein JO (2015) Bacterial Infections of the respiratory tract. en: Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant. (8 Edn) Elsevier Health, Pp 276-296.
- Ortíz-Ibarra FJ, Riega CR, Posadas LA, Arredondo GJL (1992) Infección sistémica neonatal por Streptococcus pneumoniae (reporte de un caso). Bol Med Hosp Infant Mex 46: 856-860.
- Palacios-Saucedo G, Hernández-Hernández TI, Rivera-Morales LG, Briones-Lara E, Caballero-Trejo A, et al. (2017) Infección perinatal por estreptococo del grupo B: panorama global, en América Latina y en México 153(3): 361-370.
- Rodrigo SN, Mauricio PC (2000) Chlamydia trachomatis en recién nacidos de un servicio de neonatología: Cuatro casos. Rev chil Pediatr 71(5): 423-426.
- Nissen MD (2007) Congenital and neonatal pneumonia. Paediatr Respir Rev 8(3): 195-203.
- Mussi-Pinhata MM, Nobre RA, Martinez FE, Jorge SM, Ferlin MLS, et al. (2004) Early-onset bacterial infection in Brazilian neonates with respiratory distress: a hospital-based study. J Trop Pediatr 50(1): 6-11.
- Mathur S, Fuchs A, Bielicki J, Anker J van den, Sharland M (2018) Antibiotic use for community-acquired pneumonia in neonates and children: WHO evidence review. Paediatr Int Child Health 38(Sup 1): S66-S75.
- Mathur NB, Murugesan A (2018) Comparison of Four Days Versus Seven Days Duration of Antibiotic Therapy for Neonatal Pneumonia: A Randomized Controlled Trial. Indian J Pediatr 85(11): 963-967.
- Ozdemir R, Erdeve O, Dizdar EA, Oguz SS, Uras N, et al. (2011) Clarithromycin in preventing bronchopulmonary dysplasia in Ureaplasma urealyticum-positive preterm infants. Pediatrics 128(6): e1496-e1501.
- Neu J (2020) Necrotizing Enterocolitis: The Future. Neonatology 117(2): 240-250.
- Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, et al. (2016) Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA 315(9): 889-897.
- Bell MJ, Ternberg JL, Feigin RD, J P Keating, R Marshall, et al. (1978) Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 187(1): 1-7.
- Walsh MC, Kliegman RM (1986) Necrotizing enterocolitis treatment based on staging criteria. Pediatr Clin North Am 33(1): 179-201.
- Garcia M (2020) Enterocolitis necrotizante: actualización 2020. RELAPED.
- Denning N-L, Prince JM (2018) Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol Med 24: 4 (2018).
- Dona D, Gastaldi A, Barbieri E, Bonadies L, Aluvaala J, et al. (2021) Empirical Antimicrobial Therapy of Neonates with Necrotizing Enterocolitis: A Systematic Review. Am J Perinatol DOI: 10.1055/s-0041-1730364.
- Gasque-Góngora JJ (2015) Revisión y actualización de enterocolitis necrosante. Rev Mex Pediatr 82(5): 175-185.
- Weimer KED, Smith PB, Puia-Dumitrescu M, Aleem S (2022) Invasive fungal infections in neonates: a review. Pediatr Res 91(2): 404-412.
- De Rose DU, Santisi A, Ronchetti MP, Martini L, Serafini L, et al. (2021) Invasive Candida Infections in Neonates after MajorSurgery: Current Evidence and New Directions. Pathogens 10(3): 319.
- Calley JL, Warris A (2017) Recognition and diagnosis of invasive fungal infections in neonates. J Infect 74
(Suppl 1): S108-S113. - Lausch KR, Schultz DKH, Callesen MT, Schrøder H, Rosthøj S, et al. (2019) Pediatric Candidemia Epidemiology and Morbidities: A Nationwide Cohort. Pediatr Infect Dis J 38(5): 464-469.
- Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Jillwin TJ, et al. (2020) Characteristics, outcome and Risk factors for mortality of pediatric patients with ICU‐acquired candidemia in India: a multicenter prospective study. Mycoses doi: 10.1111/myc.13145.
- Stoll B, Shane A. (2013) Recent Developments and Current Issues in the Epidemiology, Diagnosis, and Management of Bacterial and Fungal Neonatal Sepsis. American Journal of Perinatology,30:131–142.
- King J, Pana ZD, Lehrnbecher T, Steinbach WJ, Warris A (2017) Recognition and Clinical Presentation of Invasive Fungal Disease in Neonates and Children. J Pediatric Infect Dis Soc 6(suppl_1): S12-S21.
- Santolaya ME, Alvarado MT, de Queiroz Telles F, Colombo AL, Zurita J, et al. (2013) Recommendations for the management of candidemia in neonates in Latin America. Latin America Invasive Mycosis Network. Rev Iberoam Micol 30(3): 158–170.
- Pappas PG, Kauffman CA, Andes DR,Clancy CJ, Marr KA, et al. (2016) Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62(4): e1-e50.
- Bennett JE (2017) Invasive Candidiasis in Very Premature Neonates: Tiny Totswith Big Problems. Clin Infect Dis 64(7): 928–929.
- Kaufman DA (2010) Neonatal Candidiasis: Clinical Manifestations, Management, and Prevention Strategies. J Ped. (2010) 156(Suppl 2): A1–S86.
- Figueras C, Heredia CD, de García JJ, Navarro M, Ruiz-Contreras J, et al. (2011) Recomendaciones de la Sociedad Española de Infectología Pediátrica sobre diagnóstico y tratamiento de la candidiasis invasiva. Ann de Pediatr 74(5): 337.e1–337.e17.
- Arias FD, Jiménez S (2015) Manejo de la infección por candida en el recién nacido. Revista Repertorio De Medicina y Cirugía 24:123–130.
- Walsh T, Katragkou A, Chen T, Salvatore C, Roilides E (2019) Invasive Candidiasis in Infants and Children: Recent Advances in Epidemiology, Diagnosis, and Treatment. J Fungi 5(1): 11.
- Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, et al. (2012) ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18(Supply 7): 38–52.
- Arıkan K, Kalkanlı OH, Calkavur S, Akarslan K, Nuri B, et al. (2021) Safety and Effectiveness of Micafungin in Neonates: A Retrospective Analytical Study from a Tertiary Pediatric Center. Annals of Neonatology Journal 3(2): 50-69.
- Ruhnke M, Rickerts V, Cornely OA, Buchheidt D, Glöckner A, et al. (2011) Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses 54(4): 279-310.
- Rheuter T (2020) Neofax 2020.
- Juárez H, Buendía E, Lares I (2015) Pharmacology for the fetus and the newborn. Gac Med Mex 151(3): 387-395.
- Rotten CM (2016) Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr 28(2): 141-149.
- Vallejos A (2007) Reacciones adversas por antibióticos en una unidad de cuidado intensivo pediátrico y neonatal de Bogotá. Revista Biomédica 27(1): 66-75.
- WHO (2021) The importance of pharmacovigilance.
- Meyboom RHB, Hekster YA, Egberts ACG, Gribnau FWJ, Edwards IR (1997) Casual or Causal? The role of causality assessment in pharmacovigilance. Drug Saf 17(6): 374-389.
- Diario oficial de la federación (2021) NORMA Oficial Mexicana NOM-220-SSA1-2016, Instalación y operación de la farmacovigilancia.
- Aviso de sospecha de reacciones adversas a medicamentos, (2021) Comision Federal para la Protección contra Riesgos Sanitarios.
- Manual de procedimientos del departamento de farmacia hospitalaria (2011) SECRETARÍA DE SALUD INSTITUTO NACIONAL DE ENFERMEDADES RESPIRATORIAS. Available in: http://www.iner.salud.gob.mx/descargas/normatecainterna/MPdirmedica/MP_DepartamentoFarmaciaHospitalaria_15112018.pdf






























