Persistent subcutaneous nodules and Aluminium sensitization: Literature review and Case series report
Guillermo Sánchez-Rodríguez1*, Esther Serra-Baldrich1, Victoria Amat-Samaranch1, Asunción Vicente Villa2, Lluis Puig1 and Esther Roé1
1Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
2Hospital de Sant Joan de Deu, Barcelona, Spain
Submission: March 31, 2022; Published: May 24, 2022
*Corresponding author:Guillermo J Sánchez Rodríguez, Hospital de la Santa Creu i Sant Pau, Dermatology Department, Barcelona, Spain
How to cite this article:Guillermo S-R, Esther S-B, Victoria A-S, Asunción V V, Lluis P. Persistent subcutaneous nodules and Aluminium sensitization: Literature review and Case series report. Acad J Ped Neonatol 2022; 11(4): 555874. 10.19080/AJPN.2022.11.555874
Abstract
The development of persistent subcutaneous nodules at vaccination or hyposensitization injection site is a rare and under-reported event. These lesions have been attributed to the aluminium salts used as adjuvants. Patch testing on patients with the aforementioned clinical manifestations have yielded high sensitization rates to aluminium. It is believed this delayed hypersensitivity is the apparent cause of these reactive lesions in the dermis and subcutaneous tissue. In addition, aluminium hypersensitivity may cause contact dermatitis and gingivitis since it can be found in toothpaste and deodorants. We describe a series of 4 patients who developed SN at the injection site of vaccines and/or hyposensitization therapy, who underwent ultrasonography and patch testing for diagnostic confirmation. Patient management usually consists of potent topical steroids, antihistaminics, emollients and aluminium-containing products avoidance.
Keywords: Dermatology; aluminium; subcutaneous nodules;
Abbreviations: SN: subcutaneous nodules.
Introduction
The development of persistent subcutaneous nodules (SN) at vaccination or hyposensitization injection site is a rare and under-reported event [1-3]. The first series of cases of SN at injection site were published in the sixties and, three decades later, a hypothesis attributed these lesions to the aluminium salts used as adjuvants [2,4]. The evidence is scarce; thus, the incidence greatly varies among series, but a prevalence of SN at injection site of 0,5-6% has been reported [5]. Aluminium salts have been used as adjuvants for more than 80 years, as they enhance the immune response against the antigen/allergen involved [3,5-7].
Patch testing on patients with the aforementioned clinical manifestations have yielded high sensitization rates to aluminium. These increased rates point towards delayed hypersensitivity as the apparent cause of these reactive lesions in the dermis and subcutaneous tissue. In addition, aluminium hypersensitivity may cause contact dermatitis and gingivitis since it can be found in toothpaste and deodorant [1-9].
Cases report
We describe a series of 4 patients who developed SN at the injection site accompanied by itching, hypertrichosis and hyperpigmentation, whose features are summarized in (Table 1).
Non-invasive supplementary tests, such as cutaneous ultrasonography and patch testing, were employed to investigate the subcutaneous lesions and confirm the diagnostic [10].
Patient 1 was a one-year-old girl who developed a fistulized itchy nodule with serohematic exudating on the left thigh 3 months after been vaccinated against Neisseria meningitidis B with a vaccine (Bexsero®) containing aluminium hydroxide. The ultrasonography showed a fusiform hypoechoic lesion of heterogenic content in dermis and subcutaneous tissue with weak peripheral doppler sign. Patch testing could not be performed on this patient.
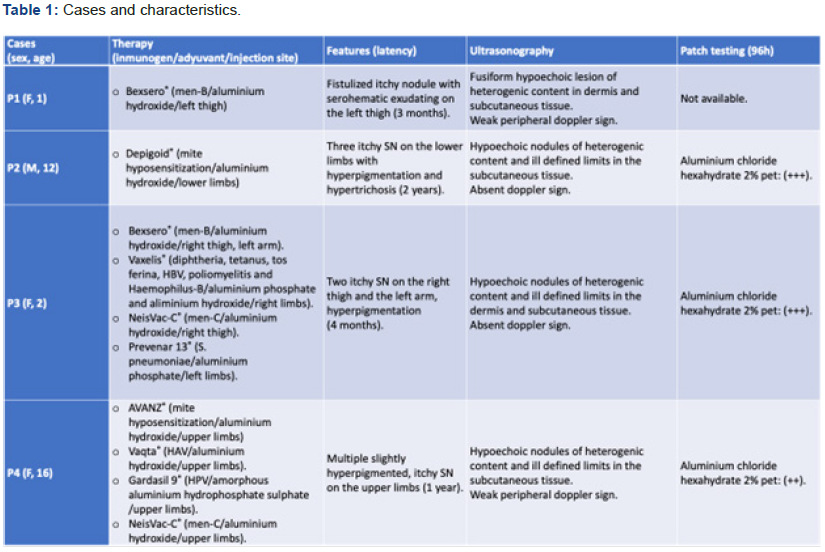
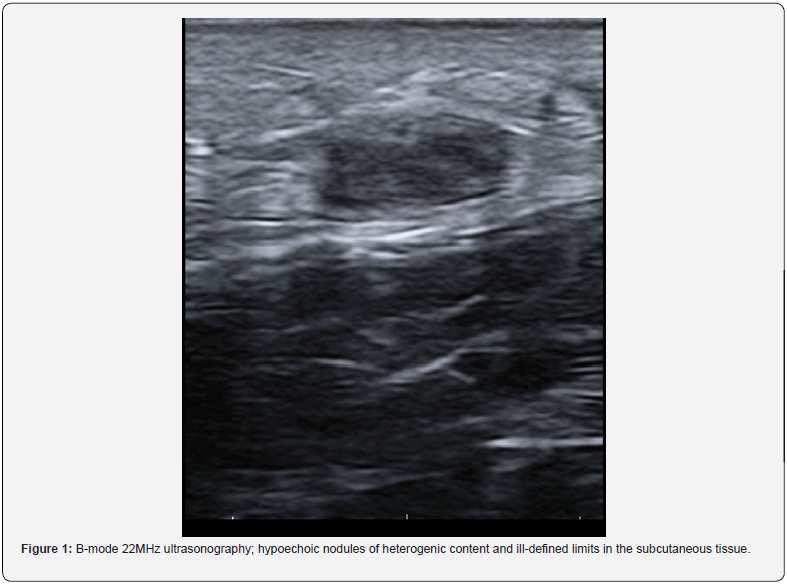
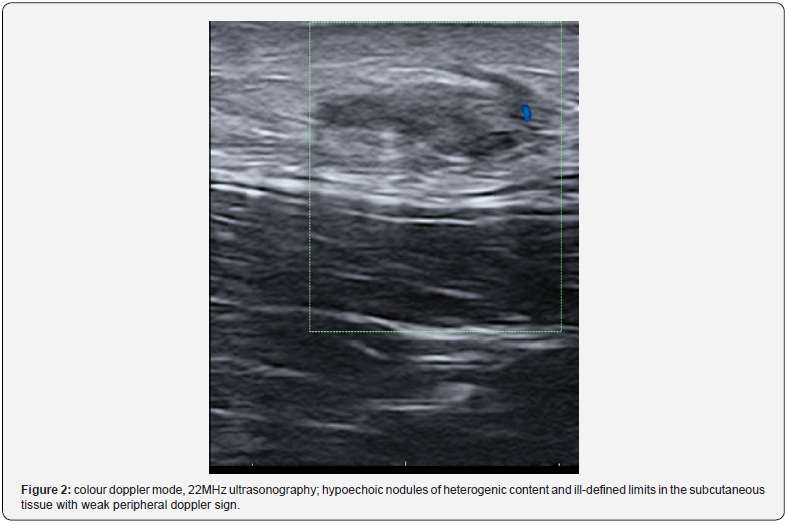
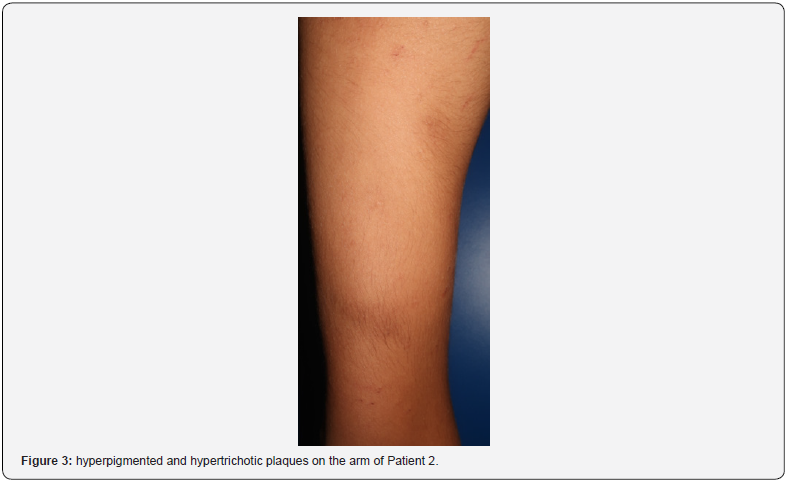

Patient 2 was a twelve-year-old boy who developed three itchy SN on the lower limbs with hyperpigmentation and hypertrichosis, 2 years after starting hyposensitization therapy against mites with Depigoid®, which contains aluminium hydroxide. The ultrasonography showed hypoechoic nodules of heterogenic content and ill-defined limits in the subcutaneous tissue, with absent doppler sign. Patch testing was positive (+++) at 96h for aluminium chloride hexahydrate petrolatum 2%.
Patient 3 was a two-year-old girl who developed two itchy SN on the right thigh and the left arm with hyperpigmentation. She consulted 4 months after receiving her latest vaccine. At that point she had received: Bexsero® (Neisseria meningitidis B) with aluminium hydroxide, Vaxelis® (diphtheria, tetanus, whooping cough, hepatitis B, poliomyelitis, and Haemophilus-B) with aluminium phosphate and aluminium hydroxide, NeisVac-C® (Neisseria meningitidis C) with aluminium hydroxide, and Prevenar 13® (Streptococcus pneumoniae) with aluminium phosphate. The ultrasonography showed hypoechoic nodules of heterogenic content and ill-defined limits in the dermis and subcutaneous tissue, with absent doppler sign. Patch testing was positive (+++) at 96h for aluminium chloride hexahydrate petrolatum 2%.
Patient 4 was a sixteen-year-old girl who developed multiple slightly hyperpigmented, itchy SN on the upper limbs. She consulted 1 year after receiving her latest injection. At that point she had received: AVANZ® (hyposensitization therapy against mites) with aluminium hydroxide, Vaqta® (Hepatitis A) with aluminium hydroxide, Gardasil 9® (human papilloma virus) with amorphous aluminium hydrophosphate sulphate, and NeisVac-C® (Neisseria meningitidis C) with aluminium hydroxide. The ultrasonography showed hypoechoic nodules of heterogenic content and ill-defined limits in the subcutaneous tissue with weak peripheral doppler sign. Patch testing was positive (++) at 96h for aluminium chloride hexahydrate petrolatum 2%.
Results
The four patients in our series presented positive results (96h: ++/+++) after patch testing for aluminium chloride hexahydrate 2% petrolatum. All of them showed superimposable findings when the ultrasonography was performed: hypoechoic nodules in the dermis and/or subcutaneous tissue with non-existent or weak doppler signal.
Besides the SN, our patients presented with itching, hypertrichosis, and hyperpigmentation. The latter are the most commonly reported signs and symptoms in literature, among others less frequent [1,2,4,5,8,9], as they were in our series.
Our patients underwent treatment with emollients and mild topical steroids resulting in clinical improvement. We also recommended avoiding aluminium containing hyposensitization therapies, non-essential vaccines, and topical products -toothpaste, deodorants-.
Discussion
Aluminium containing vaccines and hyposensitization therapies are an important source of sensitization to theses salts. This delayed hypersensitivity may be causing the SN and the signs and symptoms on the skin and producing contact dermatitis and gingivitis when aluminium containing products are applied [1-9].
Previous reports have described a clinical latency between two to thirteen months7. These findings were consistent with ours, except for Patient 2, who referred a 2-year gap between the last injection and the clinical debut.
Non-invasive techniques seem to be sufficient to archive the diagnosis without requiring scaring procedures like biopsies, especially among paediatric population [10]. Nonetheless, different histological patterns have been described in literature: panniculitis, pseudo-lymphomatous, granuloma annulare-like. The common feature amongst them is the presence of histocytes with a granular cytoplasm [5,9].
From our cohort, Patient 4 was initially clinically diagnosed with panniculitis and a deep punch biopsy was performed, whose histological pattern was also reported as panniculitis. Many screenings for primary aetiologies were performed (blood tests, autoimmunity, QuantiFERON-TB®…) that yielded normal results. This is an example of how aluminium hypersensitivity is not always an obvious diagnosis at first and why it must be reported in order to be a well know condition so it can be suspected in theses contexts.
A lower incidence of SN has been reported when intramuscular injections where performed, compared to subcutaneous ones. Aluminium avoidance and intramuscular therapies should be recommended, but they are not always available. Thus, subcutaneous nodules and/or aluminium sensitization should not be a reason to contraindicate routine vaccination [1,2,4,5,8,9]. Many authors have reported similar management when prompted with cases like ours: topical steroid creams, emollients and aluminium avoidance [1-9].
References
- Bergfors E, Björkelund C, Trollfors B (2005) Nineteen cases of persistent pruritic nodules and contact allergy to aluminium after injection of commonly used aluminium-adsorbed vaccines. Eur J Pediatr 164(11): 691-697.
- Netterlid E, Hindsén M, Björk J, Ekqvist S, Güner N, et al. (2009) There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis 60(1): 41-49.
- Andersen RM, Zachariae C, Johansen JD (2015) Aluminiumallergi og granulomer som følge af vaccination hos børn [Aluminium allergy and granulomas induced by vaccinations for children]. Ugeskr Laeger 177(18): 868-871.
- Silcock R, Crawford NW, Perrett KP (2019) Subcutaneous nodules: an important adverse event following immunization. Expert Rev Vaccines 18(4): 405-410.
- Tous Romero F, Palencia Perez SI, Rodriguez Peralto JL, F J Ortiz de Frutos (2021) Contact Allergy to Aluminum Following Vaccination: A Report of 3 Cases. Actas Dermosifiliogr (Engl Ed) S0001-7310(21)00098-3.
- Trollfors B (2020) Itching nodules and aluminium allergy following vaccination with aluminium-adsorbed vaccines-Not a negligible problem. Acta Paediatr 109(12): 2444-2445.
- González Pérez AM, Nájera Botello L, Suarez Massa D, Gullón GR, Roldán FA (2021) Sonography of subcutaneous nodules following immunization with histopathological correlation: a three-case series. J Ultrasound.
- Silcock R, Crawford NW, Selvaraj G, McMinn A, Danchin M, et al. (2020) Subcutaneous nodules following immunization in children; in Victoria, Australia from 2007 to 2016 Vaccine 38(15): 3169-3177.
- García-Patos V, Pujol RM, Alomar A, R Curell, M T Fernández-Figueras, et al. (1995) Persistent subcutaneous nodules in patients hyposensitized with aluminum-containing allergen extracts. Arch Dermatol 131(12): 1421-1424.
- Rodríguez Bandera AI, Sebaratnam DF, Feito Rodríguez M, Raúl de Lucas Laguna (2020) Cutaneous ultrasound and its utility in pediatric dermatology. Part I: Lumps, bumps, and inflammatory conditions. Pediatr Dermatol 37(1): 29-39.






























