Update for Duchenne Muscular Dystrophy - Hope for Causal Treatment of a Disorder Presenting in Early Childhood?
Gerhard Franz Walter*
International Neuroscience Institute Hannover, Germany
Submission: March 31, 2022; Published: May 11, 2022
*Corresponding author: Gerhard Franz Walter, International Neuroscience Institute Hannover, Germany
How to cite this article:Walter GF. Update for Duchenne Muscular Dystrophy - Hope for Causal Treatment of a Disorder Presenting in Early 002 Childhood?. Acad J Ped Neonatol 2022; 11(4): 555872. 10.19080/AJPN.2022.11.555872
Abstract
Duchenne muscular dystrophy (DMD) is a lethal muscle wasting disease that affects 1 in 3,600 to 6,000 males. DMD is not curable until today. In this mini-review, myoblast transplantation and stem cell therapy, read-through therapy, antisense oligonucleotide-mediated therapy, vector-mediated gene therapy, and CRISPR/Cas9-mediated gene editing are shortly addressed, approaches which alone or combined have the potential for causal treatment of DMD.
Keywords: Duchenne muscular dystrophy, myoblast transplantation, read-through therapy, antisense oligonucleotide-mediated therapy, vector-mediated gene therapy, CRISPR/Cas9-mediated gene editing
Introduction
The dystrophin gene (DMD gene) is the largest human gene. It is located on chromosome Xp21.2-p21.1 and comprises more than two million base pairs with 89 exons. Mutations in the dystrophin gene lead to a lack of dystrophin protein. “Big mutations” of the structural cytoskeletal dystrophin gene comprise deletions in which parts of the dystrophin gene are lost, or duplications in which parts of the dystrophin gene are doubled. Rarer “small mutations” are nonsense mutations with substitution of a single base-pair, thereby leading to the appearance of a stop codon where previously there was a codon specifying an amino acid; this premature stop signal terminates the production before the entire dystrophin protein is formed.
The dystrophin protein, in short dystrophin, is a subsarcolemmal rod-shaped protein with four functional domains, the actin-binding N-terminal domain, the central rod domain, the cysteine-rich domain, and the C-terminal domain [1-3]. Dystrophin is localized at the sarcolemma via the dystrophin-associated protein complex [4]. Dystrophin serves as a sort of shock absorber or stabilizer of the muscle fibers by linking the cytoskeleton to the extracellular matrix to allow the transmission of force from the contractile elements inside the cell to extracellular matrix structures, while at the same time maintaining the integrity of the muscle fibers. Binding to the sarcolemma is essential for dystrophin to protect muscle from contraction-induced injury. Its absence leads to dystrophinopathies, which were recently comprehensively reviewed [5].
Dystrophinopathies, dependent from site and extent of mutation, cover a spectrum of mild to severe dystrophies of the skeletal muscle. The effects of gene mutations are primarily determined by whether the variants disrupt the reading frame downstream of those mutations. In case of out-of-frame mutations, the open reading frame of the dystrophin gene is disrupted, leading to dystrophin deficiency and a severe phenotype presenting as Duchenne muscular dystrophy (DMD). Meanwhile, in-frame mutations preserve the reading frame, generating partially functional dystrophin protein and leading to less severe Becker muscular dystrophy (BMD). When the heart is primarily affected, an associated dilated cardiomyopathy (DCM) may occur [6]. Mild forms present with asymptomatic increase in serum concentration of creatine phosphokinase (CK) and muscle cramps with myoglobinuria.
Dystrophinopathies are inherited in an X-linked manner. The risk depends on the genetic status of the mother. Heterozygous females (carriers) have a 50% chance of transmitting DMD in each pregnancy. Sons who inherit the pathogenic variant will be affected; daughters who inherit the pathogenic variant are heterozygous and may have a range of clinical manifestations. Males with DMD usually do not reproduce. Carrier testing for atrisk females, prenatal testing, and preimplantation genetic testing are possible, if DMD in the family is known. DMD presents in early childhood and occurs in one of 3,600 to 6,000 newborn boys. Around 10% of female carriers can have symptoms of the disease. In 30% of cases, there is no known familiar predisposition.
DMD causes delays in walking independently and standing up from a supine position. Proximal weakness causes a waddling gait and difficulty climbing stairs, running, jumping, and standing up from a squatting position. DMD is rapidly progressive, with affected children being wheelchair dependent about by age 12 years. Cardiomyopathy occurs in almost all individuals with DMD after age 18 years. Few survive beyond the third decade, with respiratory complications and progressive cardiomyopathy being common causes of death due to left ventricular dilation and congestive heart failure.
The diagnosis of a dystrophinopathy should be suspected in any patient with a highly elevated creatine kinase level beyond the context of rhabdomyolysis secondary to toxic or metabolic myopathy and is established with the characteristic clinical findings and elevated CK concentration and/or, on molecular genetic testing, by identification of a hemizygous pathogenic variant in the dystrophin gene in a male and of a heterozygous pathogenic variant in a female. Females may – rarely – present with a classic dystrophinopathy or may be asymptomatic carriers. Genetic testing for dystrophinopathy is highly sensitive and specific, and identifying a proband will often lead to implications for several relatives at risk for cardiomyopathy, weakness, or anesthetic reactions [7]. The genetic testing involves a deletions/ duplications analysis performed by quantitative technique such as microarray-based comparative genomic hybridization (array- CGH), multiple ligation probe assay (MLPA). Since traditional methods for detection of point mutations and other sequence variants require high cost and are time consuming, especially for a large gene like dystrophin, the use of next-generation sequencing (NGS) has become a useful tool available for clinical diagnosis [8]. However, genetic testing of DMD identifies a certain number of variants of uncertain clinical significance (VUS). Recently, as first systematic characterization of DMD splicing variants, computational tools to prioritize VUS and a cell-based minigene assay to confirm aberrant splicing were developed, whereby rare and hitherto undetermined variants in exon and intron 10 of the DMD gene were evaluated [9].
In muscle biopsy, the affected muscle tissue undergoes necrosis of single muscle fibers, which were invaded by macrophages and cleared away. The muscle fibers are replaced by connective tissue. A lack of subsarcolemmal dystrophin can be documented (Figure 1). In the end stage of DMD, most muscle cells have been removed and, during the time, the muscle tissue is almost entirely replaced by functional fat and connective tissue (Figure 2).
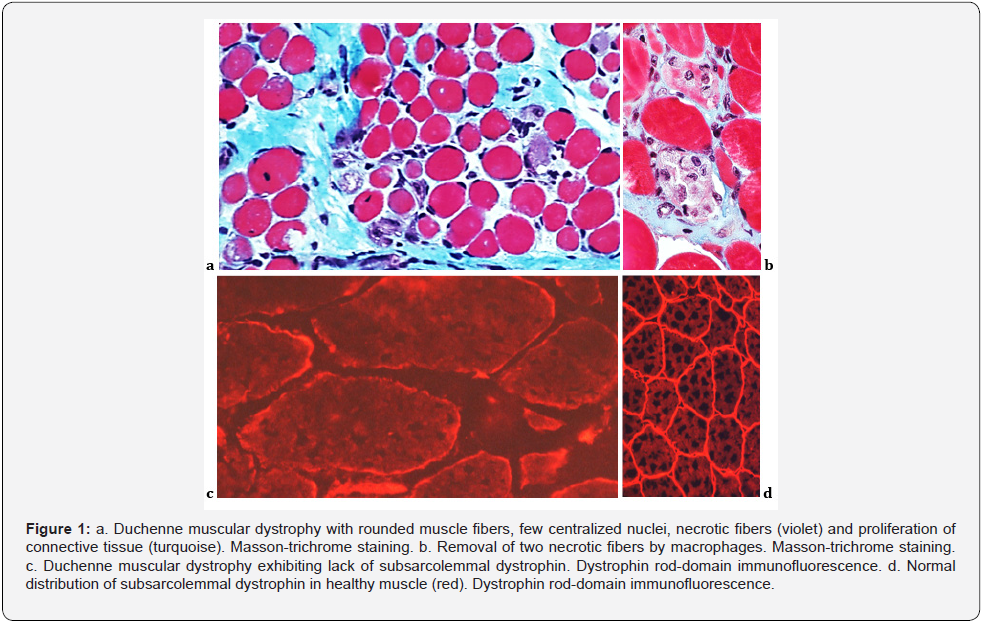
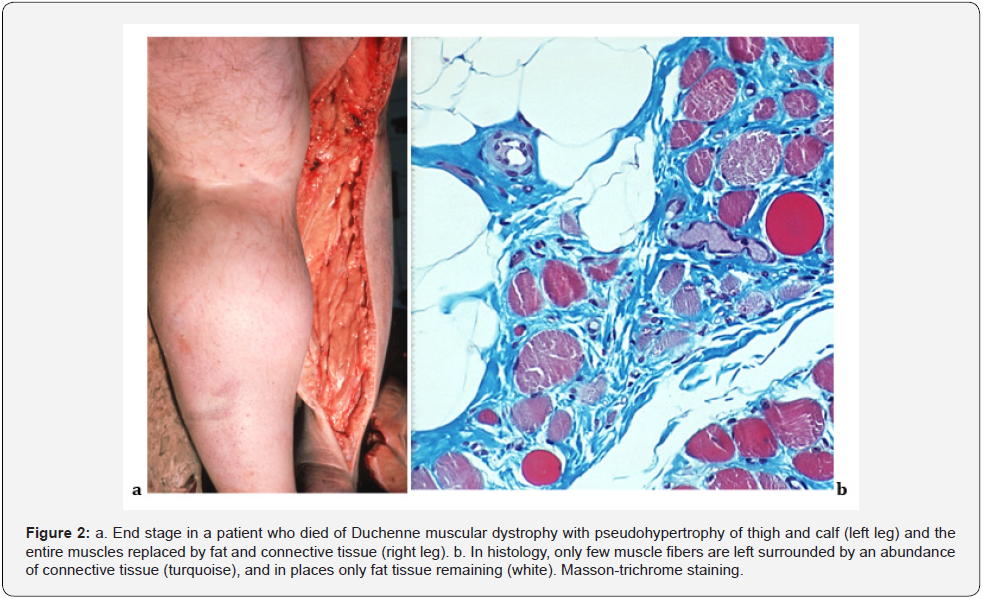
DMD is not curable until today, but it is possible to slow down the course of the disease and improve the quality of life. Management is focused primarily on supportive care, and steroid therapy is now standard-of-care. Oral corticosteroids improving muscle strength and function have changed the natural history of DMD, prolonging ambulation in affected boys between ages 5 and 15 years and reducing the risk of developing scoliosis and subsequent surgical correction, improving cardiac health, and increasing long-term survival [5,10].
Approaches to a causal treatment
Intensive research is focused on the potential for altering gene expression and gives hope for a future causal treatment of DMD. The dystrophinopathies represent model diseases to understand the personalization of genetic treatment. The many details regarding current approaches would exceed the given frame of a mini-review, therefore, two recent extensive overviews are recommended, also documenting details on current clinical trials [11-14].
Myoblast transplantation and stem cell therapy
Almost 40 years ago this still interesting cell transplantation approach was proposed. In mice, transplanted myoblasts fuse with host myofibers and express donor genes. Subsequent experiments verified the presence of dystrophin in treated animals [15,16]. However, Western blot of patient biopsies indicated a low percentage (0–5%) of dystrophin expression, which can be partially explained by immune rejection or low survival of transplanted myoblasts [17]. Human induced pluripotent stem cell (iPSC) is another tempting cell source for reconstructing dystrophic muscles. iPSCs cultured with cytokines and growth factors were committed to myogenic linage [18]. Recently, human Wharton jelly mesenchymal stem cells with high expression of Aurora kinase A were explored and might have therapeutic efficacy against DMD [19].
Read-through therapy
To treat nonsense mutations, read-through therapies utilize small molecules to interact with ribosome, which leads to insertion of an alternative amino acid at the point of premature termination codon to allow translational read-through, so that a relatively functional dystrophin protein could be generated [20].
Antisense oligonucleotide-mediated therapy
Another approach for the treatment of DMD is antisense therapy. Antisense oligonucleotides (AONs) alter pre-messenger RNA splicing to promote exon exclusion, as in eteplirsen in DMD which targets specific mutations and can be used to treat about 14% of DMD cases [21]. AON-mediated exon skipping therapy can be applied for the treatment of about 55% of DMD patients by targeting affected exons with predesigned AONs to produce a shorter but working version of dystrophin [22,23]. A new approach is the conjugation of AONs with lipophilic compounds to explore the influence of the lipophilic moiety on exon skipping efficiency in DMD [24].
Vector-mediated gene therapy
Conceptually, replacing the mutated gene with a normal one would cure the disease, but this task has encountered significant challenges due to the enormous size of the dystrophin gene and the distribution of muscle throughout the body. The former creates a hurdle for viral vector packaging and the latter begs for wholebody therapy such that all muscles are rescued. To address these obstacles, a promising approach for treating DMD is gene transfer of the highly abbreviated micro-dystrophin to restore dystrophin expression, therefore using body-wide systemic gene transfer with non-pathogenic adeno-associated viral vector (AAV). Studies in transgenic mice had revealed that highly functional microdystrophin cassettes could be generated with a size less than 4 kb [25]. Improved vectors could be generated from newly discovered AAV serotypes (such as AAV6, 8 and 9) which, when injected into the vasculature at high dose (in the range of 1014 vector genomes per kilogram) could transduce all the striated muscles in adult mice [26]. Whilst micro-dystrophin gene transfer using AAV vectors shows therapeutic success in large animal models of DMD, translating this promising therapy from bench to bedside still offers scope for many optimization steps [27,28].
CRISPR/Cas9-mediated gene editing
To obtain permanent dystrophin expression in DMD patients, gene editing, particularly CRISPR/Cas9-based gene editing, has been adopted to correct genomic deficits. The CRISPR/Cas9 system consists of the endonuclease Cas9 and a single-guide RNA (sgRNA). The Cas9 endonuclease produces DNA double-strand break at the editing sequence targeted by sgRNA. Subsequent nonhomologous end-joining leads to exon-skipping while homology-directed repair could replace dystrophin gene mutations with correct sequences and generates normal dystrophin protein [29].
Thus, adeno-associated virus (AAV)-mediated CRISPR editing holds promise to restore missing dystrophin in Duchenne muscular dystrophy (DMD). Intramuscular co-injection of Cas9 and sgRNA vectors resulted in robust dystrophin restoration in short-term studies in the mdx mouse model of DMD. Intriguingly, this strategy failed to yield efficient dystrophin rescue in muscle in a long-term (18-month) systemic injection study which was obviously caused by a selective loss of the sgRNA vector. It could be shown that the sgRNA vector dose determines the outcome of systemic AAV CRISPR therapy for DMD [30].
Conclusion
A multimodal approach might be hopeful. Sun et al. (2020) emphasize that gene therapy combined with cell transplantation and tissue engineering has the potential to lead to life-changing therapy for DMD, which possibly is also applicable for other types of muscular dystrophies including Becker muscular dystrophy and distal muscular dystrophies. In summary, there are promising novel genetically mediated therapies on the horizon, which point towards a hopeful future for individuals with DMD.
References
- Gao QQ, McNally EM (2015) The dystrophin complex: structure, function, and implications for therapy. Compr Physiol 5(3): 1223-1239.
- Zhao J, Kodippili K, Yue Y, Hakim CH, Wasala L, et al. (2016) Dystrophin contains multiple independent membrane-binding domains. Hum Mol Genet 25(17): 3647-3653.
- Chamberlain JR, Chamberlain JS (2017) Progress toward gene therapy for Duchenne muscular dystrophy. Mol Ther 25(5): 1125-1131.
- Davies KE, Nowak KJ (2006) Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol 7(10): 762-773.
- Darras BT, Urion DK, Ghosh PS (2022) Dystrophinopathies. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. PMID: 20301298.
- Kamdar F, Garry DJ (2016) Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol 67(21): 2533-2546.
- Brandsema JF, Darras BT (2015) Dystrophinopathies. Semin Neurol 35(4): 369-384.
- Falzarano MS, Scotton C, Passarelli C, Ferlini A (2015) Duchenne muscular dystrophy: from diagnosis to therapy. Molecules 20(10): 18168-18184.
- Zhang X, Chen X, Chen J, Ma Y, Huang S, et al. (2022) Functional analysis of variants in DMD exon/intron 10 predicted to affect splicing. J Hum Genet. doi: 10.1038/s10038-022-01035-y.
- Thangarajh M (2019) The dystrophinopathies. Continuum (Minneap Minn) 25(6): 1619-1639.
- Sun C, Shen L, Zhang Z, Xie X (2020) Therapeutic strategies for Duchenne muscular dystrophy: an update. Genes (Basel) 11(8): 837.
- Happi Mbakam C, Lamothe G, Tremblay JP (2022) Therapeutic strategies for dystrophin replacement in Duchenne muscular dystrophy. Front Med (Lausanne). 9: 859930.
- Chung Liang L, Sulaiman N, Yazid MD (2022) A decade of progress in gene targeted therapeutic strategies in Duchenne muscular dystrophy: a systematic review. Front Bioeng Biotechnol 10: 833833.
- Vera CD, Zhang A, Pang PD, Wu JC (2022) Treating Duchenne muscular dystrophy: the promise of stem cells, artificial intelligence, and multi-omics. Front Cardiovasc Med 9: 851491.
- Watt DJ, Morgan JE, Partridge TA (1984) Use of mononuclear precursor cells to insert allogeneic genes into growing mouse muscles. Muscle Nerve 7(9): 741-750.
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM (1989) Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 337(6203): 176-179.
- Skuk D, Tremblay JP (2003) Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil 24(4-6): 285-300.
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, et al. (2015) Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat Biotechnol 33(9): 962-969.
- Kim SJ, Park SE, Jeong JB, Oh SJ, Choi A, et al. (2022) Wharton's jelly-derived mesenchymal stem cells with high Aurora kinase A expression show improved proliferation, migration, and therapeutic potential. Stem Cells Int 2022: 4711499.
- Malik V, Rodino-Klapac LR, Viollet L, Wall C, King W, et al. (2010) Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol 67(6): 771-780.
- Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, et al. (2013) Eteplirsen Study Group. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 74(5): 637-647.
- Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, et al. (2015) The TREAT-NMD DMD Global Database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat 36(4): 395-402.
- Abreu NJ, Waldrop MA (2021) Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr Pulmonol 56(4): 710-720.
- Marchesi E, Cortesi R, Preti L, Rimessi P, Sguizzato M, et al. (2022) Antisense oligonucleotides conjugated with lipophilic compounds: synthesis and in vitro evaluation of exon skipping in Duchenne muscular dystrophy. Int J Mol Sci 23(8): 4270.
- Sakamoto M, Yuasa K, Yoshimura M, Yokota T, Ikemoto T, et al. (2002) Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem Biophys Res Commun 293(4): 1265-1272.
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, et al. (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10(8): 828-834.
- Duan D (2018) Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol Ther 26(10): 2337-2356.
- Elangkovan N, Dickson G (2021) Gene therapy for Duchenne muscular dystrophy. J Neuromuscul Dis 8(s2): S303-S316.
- Babačić H, Mehta A, Merkel O, Schoser B (2019) CRISPR-cas gene-editing as plausible treatment of neuromuscular and nucleotide-repeat-expansion diseases: a systematic review. PLoS One 14(2): e0212198.
- Wasala N, Million ED, Watkins T, Wasala LP, Han J, et al. (2022) The gRNA vector level determines the outcome of systemic AAV CRISPR therapy for Duchenne muscular dystrophy. Hum Gene Ther doi: 10.1089/hum.2021.130.






























