Association between Antenatal Care and Preterm birth: A Retrospective Study
AM Hoque, S Buckus and M Hoque
1Kwadabeka Community Health Centre, South Africa
2African College of Applied Psychology, South Africa
Submission: December 06, 2021; Published: May 06, 2022
*Corresponding author:AM Hoque Kwadabeka Community Health Centre, South Africa 4 Khuleka Drive, Clernaville, 3601, South Africaa
How to cite this article:AM Hoque, S Buckus K, M Hoque. Association between Antenatal Care and Preterm birth: A Retrospective Study. Acad J Ped Neonatol 2022; 11(4): 555871. 10.19080/AJPN.2022.11.555871
Abstract
Background: Preterm birth (PTB) remains a public health concern globally as it is one of the leading causes of morbidity and mortality in neonates. The objectives were to estimate PTB rate and to identify maternal and antenatal care associated with PTB.
Methods: A retrospective cohort study was conducted targeting all pregnant women who gave childbirths between January 2018 and December of 2019. The cross-table analysis was used to determine significance of baseline categorical data with outcome variables. The significant variables were used in binary logistic regression analysis to identify possible predictors using adjusted odds ratio (OR) and p values.
Results: A total of 1628 women gave childbirths during the study period. Preterm birth rate was 14.5%. Logistic regression output highlighted that the number of ANC visits and having birth defects were significant predictors for PTB. Women who had no ANC visit were 6.7 times, (OR=6.7, 95% CI: 2.7-16.9, p=0.000) and ANC visits (1-3) 3.4 times (OR=3.4, 95% CI: 1.8-6.6, p=0.000) more likely to have preterm deliveries. Women delivered babies with no birth defects were 76% (OR=0.24, 95% CI: 0.09-0.68, p=0.008) less likely to have preterm birth.
Conclusion: This study illustrated pregnant women who receive no or fewer ANC visits were at increased risk of PTB. Findings highlighted the need for prioritizing ANC services with counselling them on how to prevent PTB.
Keywords: Antenatal care; ANC visits; Birth defects; HIV infection; Syphilis
Introduction
The World Health Organization (WHO) defines preterm birth (PTB) as delivery of babies before 37 completed weeks of gestation [1]. In low-income countries with limited resources, over 90% of preterm babies are known to die within few days of life [2]. In 2018, the WHO highlighted that there are increasing trends of PTB over the last two decades as the incidence rates are estimated between 5% and 18% of all live births [3,4]. PTB is one of the leading causes of child death as this is reported by WHO, that 15 million babies are born preterm or prematurely every year indicating a global PTB rate of 11% and over 1 million of them die due to complications of prematurity [2]. Preterm birth is the leading cause of death among children, accounting for 18% of all deaths among children aged less than 5 years and as much as 35% of all deaths among newborns those die before 28 days of life [2]. There are significant variations of preterm birth and mortality rates between global regions, countries, and communities of a country. However, there are improved survival rates of neonates worldwide, the survival of preterm and low birthweight babies remained a challenge especially in middle- and low-income countries [5-7]. PTB is also a significant cause of short and long-term morbidity which increases the demand for neonatal care especially intensive care services. Many surviving preterm babies are known to end up with deficits such as blindness, neurological impairment and chronic respiratory problems [8]. However, the burden of preterm birth is higher particularly in low- and middle-income countries, especially in Southeast Asia and sub-Saharan Africa. The issue of preterm birth is of paramount important for achieving the United Nations (UN) Sustainable Development Goal (SDG) target, which aims to end all preventable deaths of newborns and children aged under 5 years by 2030 [3]. The true PTB burden in South Africa is unknown, however, the modelled estimates reported (2019) a rate of 12.4 per 1 000 live births [9].
A variety of factors including demographic, socioeconomic and obstetric status are reported to be associated with PTB such as maternal age, parity, previous preterm birth, multiple gestation, hypertensive disorders in pregnancy, antepartum hemorrhage, prolonged pre-labor rupture of membranes, and urinary tract infections [10,11]. A number of other maternal preexisting medical conditions have also been found to be associated with preterm births which include diabetes mellitus, urinary and genital tract infections, HIV infection and psychological stress [12]. In addition maternal the behavior and practices on antenatal care (ANC) for example numbers of ANC visits are found associated with the incidence of preterm births [13]. The ANC programme of pregnant women is known to open the opportunities to identify maternal medical, obstetrical and social risk factors so that appropriate measures may be taken in time to prevent negative pregnancy outcomes effectively and efficiently. Previous epidemiological reports have identified the associations of ANC uptakes and preterm births incidence [14-16]. A population-based study among adolescent pregnancies found a strong association between PTB with inadequate ANC coverage [17]. Furthermore, the risk of PTB is also found to be correlated with the timing of ANC initiation and the risk is higher in women who start ANC in late gestations in comparison to women who started care in the early pregnancy [18-20]. However, the earlier few reports found that there were no relationship between ANC uptake and PTB [21, 22]. The available evidence thus far is suggestive of an association between incidences of PTB and antenatal care or ANC visits. The earlier studies those reported no association may have limitations such as the studies were confined within a selected population group and the ANC package that was not well described compared to present standard of ANC package in SA [23]. The consideration of different study covariates were not included to adjust for potential confounding effects. Moreover, the number of studies from low-income countries are relatively low, with no report being available from SA thus far. Patel et al. recommended that resources should be directed to child survival strategies toward reducing the leading causes of child mortality which are PTB complications [24]. The objectives are to estimate preterm delivery rate and to identify risk factors.
Methods
Setting and population
Kwadebeka community health center (KCHC) is a primary health care (PHC) facility running midwife obstetric unit (MOU) for delivering pregnant women for the people living in the communities of Kwadebeka and Clermont, the residence of over 150,000 predominantly black people. These communities are situated within the municipal boundaries of eThekweni (Durban) Metropolitan city. The City of Durban is featuring the South Africa’s largest port and is situated in the province of KZN. Most of the residences are known poor as they are unemployed, living mainly in informal types of dwellings and reliant on public health services at KCHC as a first contact for healthcare based on the principles of District Health System implemented in1996 after democratization of SA. Full PHC services package including maternity services are available at KDC is available 24 hours a day. The main functions of the MOU (according to national guidelines) are to: provide antenatal care for low- and intermediate- risk pregnant women, treatment of common problems of pregnancy, management of labor and delivery services for low-risk women and postnatal check-ups. The management of emergencies during antenatal care and delivery services and referral to appropriate hospitals are also undertaken at the MOU [23]. During daytime (7 am to 4 pm) there are 3 and afterhours (4 pm to 7 am) 2 midwives allocated together with other support staff to conduct deliveries and care for mothers and newborns. Antenatal care and delivery services are rendered at KCHC according to the national protocol and guidelines developed and implemented since 2002 [23]. During antenatal care and at the time of delivery the pregnancy complications identified and referred to hospitals are not included in this study. The antenatal and delivery conditions for referral are listed and in accordance with the national guidelines [23].
Study design, sample selection and data collection
A register based retrospective cohort study targeting all pregnant women who had childbirths at MOU of KCHC between January of 2018 and December of 2019 was conducted. Women who delivered at home or on the way to the MOU (known as BBA or birth before arrival) were registered for postnatal and baby care. Data was collected from the labour ward “birth register” the only official document for all childbirths at the facility. The register contains among others the age, gravidity, parity, history of ANC during pregnancy, gestational ages (GA) in weeks at ANC initiation (booking for ANC) and delivery, number of ANC visits), HIV and syphilis infection status and delivery outcomes (live births, stillbirths) of women. The dependent variable for this study was preterm deliveries. Preterm delivery was considered when mothers delivered a baby between 28 weeks and 36 weeks of GA or above 1000 grams body weight. The term delivery was considered between 37 and 41 weeks of GA. Any delivery that occurred at 42 completed weeks GA or afterwards was considered as post term delivery. The GA was confirmed with the first day of last menstruation (LMP) as standard practice in obstetrics. In cases the LMP unknown, the symphysis pubis to fundal heights (SPF) at the booking visits measured in centimeters or ultrasonography measurements of GA were considered [23].
Data analysis
Data were entered into Excel 365 (Microsoft, USA) from manual birth register and then exported and coded for analysis in SPSS 22.0.1 (SPSS Inc, Chicago, IL, USA). Descriptive statistics were used to describe continuous and categorical variables. Both the mean and standard deviation (SD) values were calculated for continuous and numbers or proportions for categorical variables. Age was categorized into < 20 (teenage), 20-24, 25-29, 30- 34,35-39 and >40 years; parity into 0, 1-2, 3-4 and >5, number of antenatal visits into 0, 1-3, 4-7 and > 8 visits a having birth defects, HIV and syphilis status of mothers at birth. Categorical variables were assessed using crosstabs. The dependent variables preterm birth was recoded as binominal variables and defined as follows: PTB as GA < 37 (yes=1) vs. ≥37 (no=0) weeks. Gestational age was calculated using the last menstrual period (LMP) or SFH or using ultrasound estimation. Crosstabs were used to explore associations of outcome variable. Differences in proportions between variables were tested with the Pearson’s chi-square (X2) test and p values. The significant variables (obtained from crosstable) were used in binary logistic regression analysis to identify possible predictors for outcome variables. For regression models, the results were expressed as adjusted odds ratios (OR) with 95% confidence intervals (95% CI) and associated p-values. P-values were reported to three decimal places with values less than 0.001 reported as <0.001.
Ethical consideration
Prior permission to conduct the study was obtained from the Umgungundlovu Health Ethics Review Board (Reference no. UHERB 015/2020). Further permission was sought from the Research Committee of the KZN Health. Additional permission was obtained from the management of the KCHC to use the relevant data for the study. Secondary data were used, and hence informed consent was waived.
Results
The number of women gave childbirths during the study was 1628. (Table 1) illustrated the baseline information of the study sample. Teenage women constituted 14.7% and age group between 20-29 years was 57.5%. Pregnancy with advanced age (> 35 years) was only 8.3%. The mean age of these mothers were 26 years with SD of 5.7 years indicating a younger population. The minimum and maximum ages ranged between 14 to 43 years. Primigravid women were 30.2%, however, over half (56.1%) of them had parity between 1 and 2 and a few (1.3%) had parity > 5. BBA rate was 4%, over half (52.4%) had ANC before 20 weeks of gestation. Mothers did not have any ANC was low of 6.2%. Mothers delivered babies with any birth defects were 1%. HIV and syphilis infection rate were 45.1% and 2.2% at delivery respectively. The preterm delivery rate was 14.5%. Cross table analysis (Table 2) showed that the higher rate (59.8%) of preterm delivery was among those women initiated ANC after 20 weeks of GA (p<0.001). The other variables showed significantly different PTB rates were those women who did not initiate ANC at all, number of ANC visits, those had BBA and those had delivered babies with birth defects (p<0.05). Age, parity, HIV and syphilis infections were not significant for PTB. Logistic regression output (Table 3) highlighted that number of ANC visits and having birth defects were the predictors for preterm delivery. Women who had no ANC visit 6.7 times, (OR=6.7, 95% CI: 2.7-16.9, p=0.000) and lower number of ANC visits (1-3), 3.4 times (OR=3.4, 95% CI: 1.8-6.6, p<0.001) more likely to have preterm births. Having no birth defects of babies were 76% (OR=0.24, 95% CI: 0.09-0.68, p=0.008) less likely to have preterm birth.
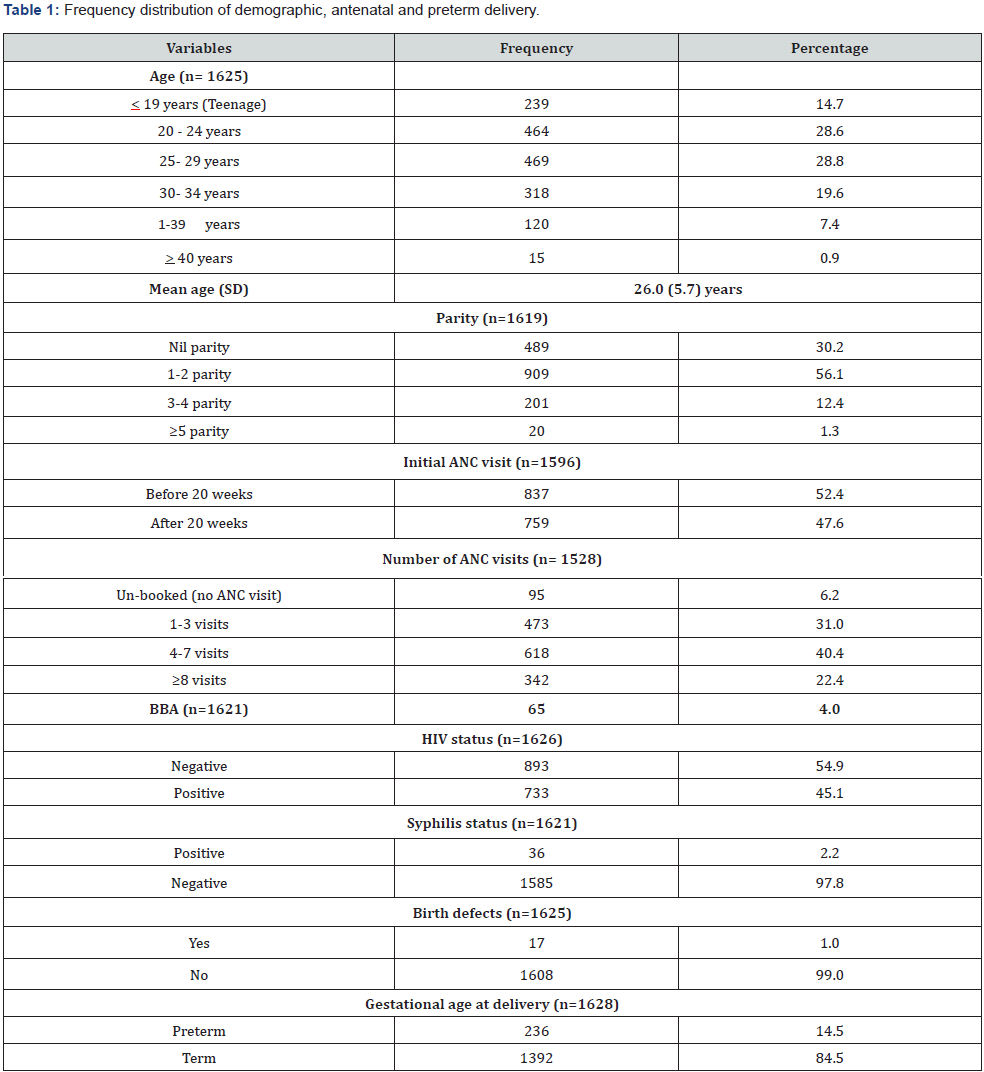
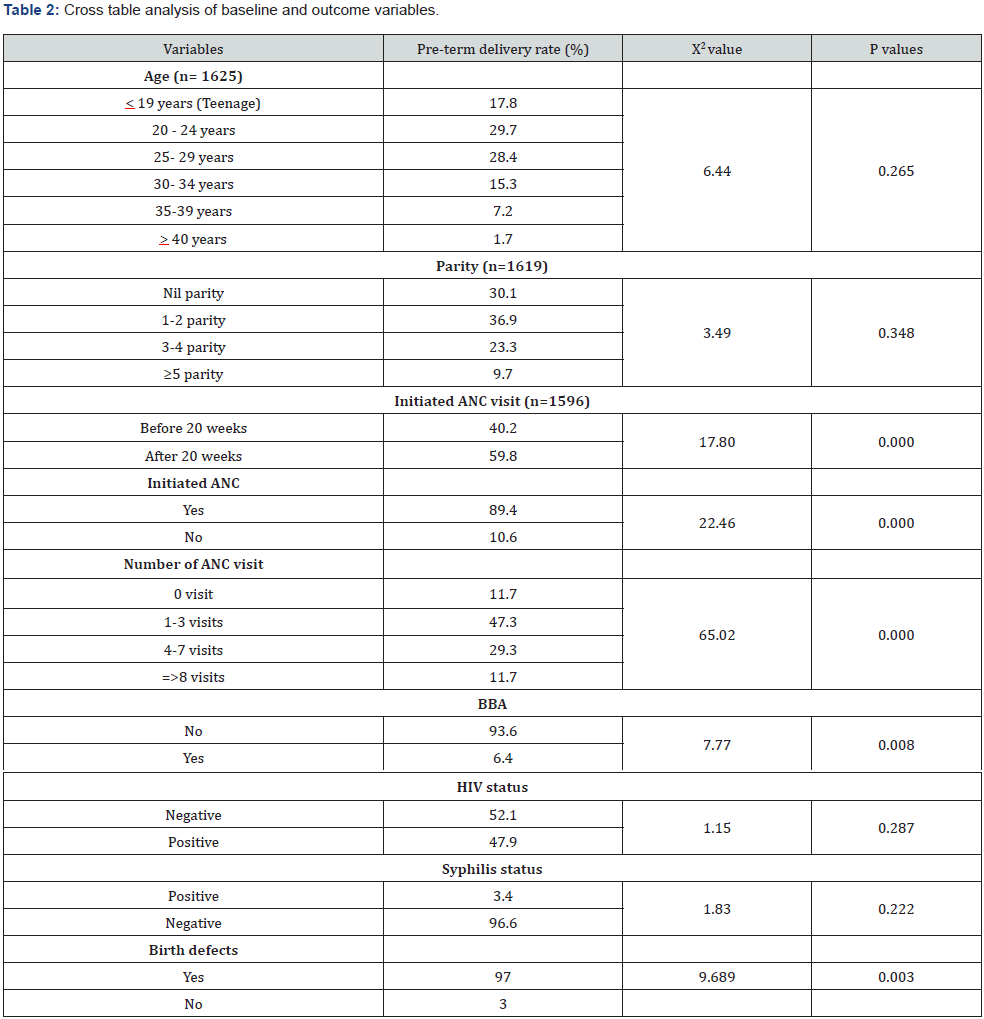
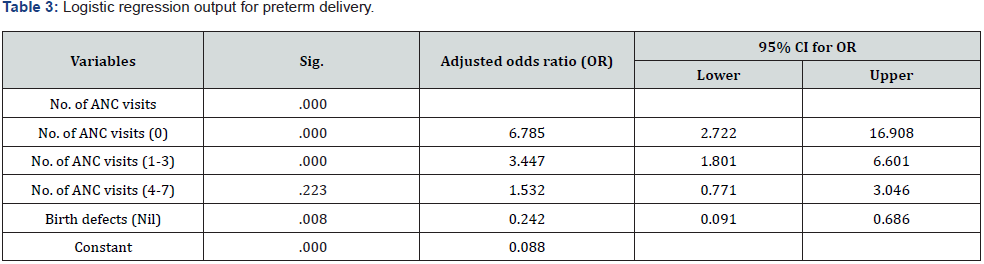
Reference group: ANC visits >8, birth defect (yes).
Discussion
There are not enough studies in SA evaluating the association of ANC utilization and preterm birth in SA. Preterm birth is one of the most important public health problems in SA and worldwide. In our study, we observed a higher rate (15.4%) of preterm births than the rate estimated (12.4%) for SA in 2019 [9]. However, our PTB rate is higher similar to the rates 16.4% and 17%% reported from Durban and Cape Town respectively with similar settings [25, 26]. We found the number of ANC visits is a strong predictor for preterm birth. The women who attended more ANC visits have fewer possibilities of having preterm births irrespective of the quality of ANC care. Provisions for easy accessibility in receiving adequate ANC during the course of pregnancy undoubtedly prevent adverse neonatal outcomes, such as preterm deliveries. Research has indicated that pregnant women who attend antenatal visits are at lower risk for having preterm deliveries than those women who deliver before accessing the health facility [27]. In our study we found that the number of ANC visits is significantly associated with preterm deliveries in a dose-dependent way. Women who had no ANC visits were 6.7 times, and ANC visits [1-3] were 3.4 times more likely to have preterm deliveries. Our findings are similar to the findings of a study conducted in rural Bangladesh where preterm deliveries was 2.4 times higher among women who received ≤1 ANC compared with women who received ≥3 ANC [13]. Our findings also concur with findings in an observational study conducted on over nine million women in China, where an increase in the frequency of ANC visits significantly reduced the risk of preterm deliveries [28]. The reduction of preterm births with increased ANC visits resulted from better access to ANC attendance with consistent pregnancy follow-up and the prompt identification and management of complications. The study in China, between 2012 and 2018, further reported a 41% increase in women with seven or more ANC visits, and 64% decrease in women with zero to three ANC visits and 40% decrease in those pregnant women who had four to six ANC visits, and these indicators mitigate the high preterm delivery rate in China [28].
Our findings of the number of ANC visits and preterm deliveries differ from findings of other studies that found no significant association between the number of ANC visits alone and preterm deliveries, however it may be the content and timing of ANC visits and preterm deliveries that was significantly associated [20]. One plausible explanation for the above difference observed, is that the above studies were conducted in high income countries, where comparable differences in the content of ANC will exist between high- and low-income country-settings. It is thus evident that more research on the risks and association of preterm birth and the number of ANC visits and the evaluation of quality and timing of ANC in relation to preterm deliveries needs to be conducted. Our study found a statistically significant association with preterm births and birth defects. A study from the USA has demonstrated that birth defects are twice likely to occur in those visits babies born prematurely as compared to babies born at term, of which birth defects were found in approximately 8% of babies born preterm [29]. In that study the most common birth defects were the central nervous system and cardiovascular defects. An extremely large population-based study has found a significant association with preterm births and brain defects [30]. It is still unclear as to the extent and part in which birth defects occur in preterm births and thus more research on this topic will prove beneficial in better understanding. Although assumptions are made that infections including HIV are associated with adverse birth outcomes including preterm birth, the present study did not find any significant association between maternal syphilis and HIV with preterm births and concurs with the findings of cohort studies from Malawi and SA those demonstrated no association between HIV infection and maternal syphilis and preterm births [25, 31]. However, the study in Malaria differed from our study as the pregnant women in Malawi were naïve to antiretroviral therapy. But the study from SA found that most of the HIV infected mothers were on ART [25]. A study conducted in Ethiopia found a significant association between preterm births and a positive HIV status [32]. A two-fold increase of preterm births was reported in HIV positive women in a study in Kenya and a study in KwaZulu Natal reported that mothers who are HIV positive, are four times more likely to have a preterm birth [33, 34].
Another study in Tanzania further mentioned that a risk factor for preterm births was found in women with HIV stage two or greater [35]. One plausible explanation for this non-significant association between the HIV status and preterm births in our study could be the use of antiretroviral therapy (ART) used by these pregnant mothers to manage their HIV. Various studies have concluded that women on ART are at a higher risk of delivering before term, whilst another study found an increased rate of preterm birth in women using ART during pregnancy, however these results were not significant [36-39]. On the contrary, a recent large cohort study in USA found that the use of ART was significantly associated with a reduction in preterm births [40]. The estimated worldwide maternal syphilis rate in 2016 was 0.69% and responsible for 41 000 preterm or low birth weight deliveries [41]. A study in China found that babies born to mothers infected with syphilis are 1.5 times more likely to be born preterm [42]. However, a retrospective study conducted in Australia agree with our findings and demonstrated no statistical association between maternal syphilis and higher rates of preterm birth [43]. It has been found that timeous detection and management of maternal syphilis is an extremely beneficial intervention in reducing adverse birth outcomes, including but not limited to preterm births [43,44,45]. It is likely that the reason for a nonsignificant association between maternal syphilis and preterm births in the present study is that only 3.4% of the study population tested positive for syphilis (at birth not at initiation of ANC), of which majority (88.3%) of the pregnant women had at least one ANC visit. All pregnant mothers are screened at their initial ANC visit for syphilis and if found positive are treated accordingly. Early detection in pregnancy and the appropriate treatment translates to a low syphilis-seropositive conversion rate as was found in a study where adverse birth outcomes were found in pregnant women infected with syphilis that received treatment only after 28 weeks gestation [46].
Strengths and weaknesses
Although vast majority (more than 95%) of pregnant women were known to attend public health care facilities for antenatal care in rural areas of KZN, exclusion of deliveries at home and in private hospitals are considered as a limitation of the present study. The retrospective review of records limited the availability of some other important variables and consequently led to information bias.
Conclusion
This study illustrates a higher rate of preterm delivery rate like other recent studies from SA of similar settings with an association of ANC and number of ANC visits. Pregnant women who receive no or few ANC visits are at a significant risk of adverse birth outcomes, particularly of PTB. Furthermore, this study demonstrates that babies born with birth deformities are also at a higher risk of being born premature. The importance of consistent ANC and ANC visits will allow for timeous identification and prompt management of potential complications during the course of pregnancy, inevitably reducing the high rates of PTB. Findings highlight the need for prioritizing maternal health services in low socio-economic communities. It is fundamental that policy makers ensure that strategies are in place to reduce adverse birth outcomes.
Recommendation
Efficient maternal health services with an increased uptake of ANC visits by pregnant women and quality of ANC services will reduce the burden of adverse pregnancy outcomes particularly in low-resource settings. It is recommended that a survey be conducted on pregnant women attending KCHC on their perception and knowledge of ANC. This will merit in identifying, evaluating, and addressing the challenges experienced in accessing quality ANC. Furthermore, we advocate for both improving our communication to pregnant women and strengthening our education program on all the benefit of ANC and to reduce preterm births and other possible adverse pregnancy outcomes.
Authors’ contributions
AMH has designed and developed the research concept, protocol submission for ethical approval, involved in supervision of data collection, statistical analysis and preparation of the final manuscript. SB involved in study design, data collection, drafting and editing the manuscript. MH for data capture, data coding, data analysis and drafting of the manuscript. All authors read and approved the final manuscript.
References
- (1977) WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand 56(3): 247-253.
- Liu L, Oza S, Hogan D, Chu Y, Perin J, et al. (2016) Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388(10063): 3027-3035.
- (2019) World Health Organization. Newborns: reducing mortality. World Heal Organ: 21.
- Haas DM (2011) Preterm birth. BMJ clinical evidence 1404.
- Basu S, Rathore P, Bhatia BD (2008) Predictors of mortality in very low birth weight neonates in India. Singapore Med J 49(7): 556-560.
- Afjeh SA, Sabzehei MK, Fallahi M, Esmaili F (2013) Outcome of very low birth weight infants over 3 years report from an Iranian center. Iranian journal of pediatrics 23(5): 579-587.
- Rhoda NR, Gebhardt GS, Kauchali SBP, S Kauchali, P Barron (2018) Reducing neonatal deaths in South Africa. : Progress and challenges. The South African Medical Journal 108 (3a): S9-s16.
- Wisanskoonwong P (2012) Midwifery primary health care groups during childbearing. Lismore, NSW: Southern Cross University.
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, et al. (2019) Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet. Global health 7(1): e37-e46.
- Wagura P, Wasunna A, Laving A, Wamalwa D, Ng'ang'a P (2018) Prevalence and factors associated with preterm birth at kenyatta national hospital. BMC pregnancy and childbirth 18(1): 107.
- Althabe F, Moore JL, Gibbons L, Mabel Berrueta, Shivaprasad S Goudar, et al. (2015) Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network’s Maternal Newborn Health Registry study. Reprod Health 12: S8.
- Blencowe H, Cousens S, Chou D, Mikkel Oestergaard, Lale Say, et al. (2013) Born Too Soon: The global epidemiology of 15 million preterm births. Reprod Health 10: S2.
- Pervin J, Rahman SM, Rahman M, Aktar S, Rahman A (2020) Association between antenatal care visit and preterm birth: a cohort study in rural Bangladesh. BMJ open 10(7): e036699.
- Blondel B, Dutilh P, Delour M, Uzan S (1993) Poor antenatal care and pregnancy outcome. European Journal of Obstetrics & Gynecology and Reproductive Biology 50(3): 191-196.
- Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA (2002) The impact of prenatal care in the United States on preterm births in the presence and absence of antenatal high-risk conditions. Am J Obstet Gynecol 187(5): 1254-1257.
- Zhang B, Yang R, Liang SW, Wang J, Chang JJ, et al. (2017). Association between prenatal care utilization and risk of preterm birth among Chinese women. J Huazhong Univ Sci Technolog Med Sci 37(4): 605-611.
- Debiec KE, Paul KJ, Mitchell CM, Hitti JE (2010) Inadequate prenatal care and risk of preterm delivery among adolescents: a retrospective study over 10 years. Am J Obstet Gynecol 203(2) 122 e1-e6.
- Asundep NN, Jolly PE, Carson A, Turpin CA, Zhang K, et al. (2014) Antenatal care attendance, a surrogate for pregnancy outcome? The case of Kumasi, Ghana. Matern Child Health J 18(5): 1085-1094.
- Barros H, Tavares M, Rodrigues T (1996) Role of prenatal care in preterm birth and low birthweight in Portugal. J Public Health Med 18(3): 321-328.
- Beeckman K, Louckx F, Downe S, Putman K (2013) The relationship between antenatal care and preterm birth: the importance of content of care. Eur J Public Health 23(3): 366-371.
- Kramer MS (1987) Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 65(5): 663-737.
- McDuffie RS, Beck A, Bischoff K, Cross J, Orleans M (1996) Effect of frequency of prenatal care visits on perinatal outcome among low-risk women A randomized controlled trial. JAMA 275(11): 847-851.
- National Department of Health South Africa (2016) Guidelines for Maternity Care in South Africa—A Manual for Clinics, Community Health Centers, and District Hospitals. Department of Health, Pretoria, South Africa 4th [Ed’s].
- United Nations Children’s Fund South Africa (UNICEF SA-2011). Improving Newborn Care in South Africa, Lessons learned from Limpopo Initiative for Newborn Care (LINC). Pretoria.
- Jeena PM, Asharam K, Mitku AA, Naidoo P, Naidoo RN (2020) Maternal demographic and antenatal factors, low birth weight and preterm birth: findings from the mother and child in the environment (MACE) birth cohort, Durban, South Africa. BMC pregnancy and childbirth 20(1): 1-11.
- Zar HJ, Pellowski JA, Cohen S, Barnett W, Vanker A, et al. (2019) Maternal health and birth outcomes in a South African birth cohort study. PloS one 14(11): e0222399.
- Requejo J, Merialdi M, Althabe F, Keller M, Katz J, et al. (2013) Born Too Soon: Care during pregnancy and childbirth to reduce preterm deliveries and improve health outcomes of the preterm baby. Reproductive health 10(1): 1-15.
- Deng K, Liang J, Mu Y, Liu Z, Wang, Y, et al. (2021) Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Global health 9(9): e1226-e1241.
- Honein MA, Kirby RS, Meyer RE, Xing J, Skerrette NI, et al. (2009) The association between major birth defects and preterm birth. Matern Child Health J 13(2): 164-175.
- Brown WR (2009) Association of preterm birth with brain malformations. Pediatr Res 65(6): 642-646.
- van den Broek NR, Jean-Baptiste R, Neilson JP (2014) Factors Associated with Preterm, Early Preterm and Late Preterm Birth in Malawi. PLoS ONE 9(3): e90128.
- Gebreslasie K (2016) Preterm birth and associated factors among mothers who gave birth in Gondar town health institutions. Advances in Nursing.
- Ravindran J, Richardson BA, Kinuthia J, Unger JA, Drake AL, et al. (2021). Chlamydia, Gonorrhea, and Incident HIV Infection During Pregnancy Predict Preterm Birth Despite Treatment. J Infect Dis 224(12): 2085-2093.
- Naidoo M, Sartorius B, Tshimanga-Tshikala G (2016) Maternal HIV infection and preterm delivery outcomes at an urban district hospital in KwaZulu-Natal 2011. Southern African Journal of Infectious Diseases 31(1):25-28.
- Zack RM, Golan J, Aboud S, Msamanga G, Spiegelman D, et al. (2014) Risk factors for preterm birth among HIV-infected Tanzanian women: a prospective study. Obstetrics and gynecology international 11(5): 261689.
- Venkatesh KK, Farhad M, Fenton T, Moodley D, Naik S, et al. (2019). Association between HIV antiretroviral therapy and preterm birth based on antenatal ultrasound gestational age determination: a comparative analysis. AIDS 33(15): 2403-2413.
- Chetty T, Thorne C, Coutsoudis A (2018) Preterm delivery and small-for-gestation outcomes in HIV-infected pregnant women on antiretroviral therapy in rural South Africa: Results from a cohort study, 2010-2015. PloS one 13(2): e0192805.
- Mesfin YM, Kibret KT, Taye A (2016) Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod Health 13: 30.
- Gagnon LH, MacGillivray J, Urquia ML, Caprara D, Murphy KE, et al. (2016) Antiretroviral therapy during pregnancy and risk of preterm birth. Eur J Obstet Gynecol Reprod Biol 201: 51-55.
- Venkatesh KK, Edmonds A, Westreich D, Dionne-Odom J, Weiss DJ, et al. (2021) Associations between HIV, antiretroviral therapy and preterm birth in the US Women’s Interagency HIV Study, 1995–2018: a prospective cohort. HIV Medicine 23(4): 406-416.
- Korenromp EL, Rowley J, Alonso M, Mello MB, Wijesooriya, NS, et al. (2019). Global burden of maternal and congenital syphilis and associated adverse birth outcomes-Estimates for 2016 and progress since 2012. PloS one 14(2): e0211720.
- Wan Z, Zhang H, Xu H, Hu Y, Tan C, et al. (2020) Maternal syphilis treatment and pregnancy outcomes: a retrospective study in Jiangxi Province, China. BMC pregnancy and childbirth 20(1): 648.
- Burton AE, Thomas S (2019) Sexually transmitted infections and preterm birth among Indigenous women of the Northern Territory, Australia: A case-control study. Aust N Z J Obstet Gynaecol 59(1): 147-153.
- Blencowe H, Cousens S, Kamb M, Berman S, Lawn J E (2011) Lives Saved Tool supplement detection and treatment of syphilis in pregnancy to reduce syphilis related stillbirths and neonatal mortality. BMC Public Health 11: S9.
- Curry SJ, Krist, AH, Owens DK, Barry MJ, Caughey AB, et al. (2018) Screening for Syphilis Infection in Pregnant Women: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 320(9): 911-917.
- Liu H, Chen N, Yu J, Tang W, He J, et al. (2019) Syphillis-attributable adverse pregnancy outcomes in China: a retrospective cohort analysis of 1187 pregnant women with different syphilis treatment. BMC Infect Dis 19(1): 292.






























