A Low-Cost Venturi Ambient Air-Oxygen Blender for Neonatal Oxygen Therapy
Kamyar Mollazadeh-Moghaddam1,2,3, Thomas Friedrich Burke1,3,4,5,6,ǂ, Michelle Dundek1,3,ǂ, Shu Ho Yeung2, Rupam Sharma1,3, Revathi Ravi1,6 and Anuj Bellare2,3*
1Division of Global Health Innovation, Department of Emergency Medicine, Massachusetts General Hospital, Boston, USA
2Orthopedic Nanotechnology Laboratory, Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, USA
3Vayu Foundation, Boston, USA
4 Harvard Medical School, Boston, USA
5 Harvard TH Chan School of Public Health, Boston, USA
6 Department of Pediatrics, Massachusetts General Hospital for Children, Boston, USA
Submission: January 09, 2020; Published: February 04, 2020
*Corresponding author: Anuj Bellare, Orthopedic Nanotechnology Laboratory, Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, Massachusetts, USA ǂCo-second authors: Thomas Friedrich Burke, Division of Global Health Innovation, Department of Emergency Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA Michelle Dundek, Division of Global Health Innovation, Department of Emergency Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA
How to cite this article: Kamyar M-M, Thomas F B, Michelle D, Shu H Y, Rupam S, et al. A Low-Cost Venturi Ambient Air-Oxygen Blender for Neonatal Oxygen Therapy. Acad J Ped Neonatol. 2020; 9(1): 555808. DOI: 10.19080/AJPN.2020.08.555808
Abstract
The concentration of oxygen delivered to neonates in respiratory distress should be controlled to prevent hyperoxia. Current available oxygen blending devices have limited use in resource-limited settings due to their reliance on electricity, compressed air, skilled maintenance, and high cost. This study evaluated the ability of a novel blending device that addresses these limitations to deliver inspired concentrations of oxygen over a range of 30-100%. Our blending device was designed based on the Venturi principle. The blender consists of a nozzle, air entrainment window, and orifice. Oxygen exits the nozzle at high velocity into an air-entrainment chamber, where the low pressure surrounding the jet draws in ambient air. The mixture of air and oxygen is then transported into the orifice and thereafter further downstream via tubing. We investigated the effect of geometric factors and process variables on the delivered oxygen concentrations. The diameter of the Venturi nozzle and outlet orifice, the cross-sectional area of the air-entrainment window, the distance between the Venturi nozzle and the outlet orifice, flow rate, and temperature were each analyzed as independent variables. Understanding the geometric relationships between Venturi nozzle diameters, air-entrainment window cross-sectional areas, and Venturi nozzle to outlet orifice distances provided guidance on the design of an ultra-low-cost Venturi ambient air-oxygen blender. This study demonstrates the feasibility of manufacturing an air-oxygen blender that is low cost, does not require electricity or compressed air, and can provide accurate concentrations of oxygen for optimal delivery to neonates with respiratory distress.
Keywords:Global health; Affordability; Accessibility of health services; Healthcare quality; Oxygenation; Hyperoxia; Neonatal diseases; Medical device design; Biomedical engineering; Respiratory distress and failure
Abbreviations: CPAP: Continuous Positive Airway Pressure; FiO2: Fractional Oxygen Concentration
Introduction
Supplemental oxygen is often necessary for preterm newborns in respiratory distress [1]. Nasal cannulas (with low flow or high flow gas), different types of continuous positive airway pressure (CPAP), nasal intermittent positive pressure ventilation, hoods, and facemasks are common noninvasive devices used to deliver supplemental oxygen to neonates [2-7]. While supplemental oxygen is often important for these patients, high concentrations (hyperoxia) may be harmful [8]. Bronchopulmonary dysplasia and retinopathy of prematurity are two major adverse effects of administering high concentrations of inspired oxygen to preterm neonates [9,10]. Different types of oxygen blending devices are used in high-resource settings to regulate concentrations of oxygen and prevent hyperoxia. WHO recommends initial fractional oxygen concentrations (FiO2) of 30% when using continuous positive airway pressure devices (CPAP) for premature newborns, and only increasing FiO2 when absolutely necessary [11]. There generally are two types of blenders to control FiO2: 1/valve blenders which are used for neonates and older patients and 2/Venturi blending devices which are used for oxygen therapy in older patients. Valve devices have sources of air and oxygen at elevated pressures, and concentrations are adjusted by changing the relative flow of each gas. The most common blenders are valve devices that mix compressed oxygen and compressed air from individual sources by adjusting a knob without electricity. However, compressed air is difficult to find in low-resource settings and these types of blenders are expensive [12].
Other types of valve blenders use pumps for blending, such as in the Pumani and Diamedica Baby CPAP devices. Pumani devices supply oxygen from an oxygen source while Diamedica devices use electricity to supply oxygen through an oxygen concentrator. While these devices do not require compressed air, reliance on uninterrupted electricity and relatively high cost are important limitations [13,14]. Venturi blending is another mechanism to blend oxygen and air. In Venturi blenders a jet of pure oxygen is ejected from a nozzle through an open window. Based on the Venturi principle, a low-pressure environment develops immediately around the jet which entrains ambient air before the jet of mixed gas is recaptured on the other side of the window. Venturi blenders do not use compressed air or pumps, however many (e.g. MaxVenturi, Whisper Flow) are expensive [15]. Venturi masks use a similar mechanism to blend and they are low cost yet cannot be attached to high resistance systems such as CPAP devices [16]. Additionally, while Venturi devices do not use electricity or compressed air to blend oxygen and air, they have not yet been designed for use in neonates. The lack of low-cost oxygen blender device designs for neonates and the need for electricity and compressed air are barriers to blending oxygen for neonatal therapies such as CPAP in low-resource settings [17-25]. Even if hospitals in low-resource settings were able to purchase existing blenders, most would not be able to use them since uninterrupted electricity and compressed air are scarce. In sub-Saharan Africa two thirds of health facilities do not have access to uninterrupted electricity, making pump blenders impractical and unsafe [26]. Use of pure oxygen leads to hyperoxia and potential complications of lungs, brain and eyes. A few clinicians in low-resource settings have improvised their own blenders. For example, Daga, Joshi, Gunjal, and Mhatre used an aquarium pump to provide air, but this innovative approach is limited by its dependency on electricity and inability to regulate concentrations of oxygen accurately [27]. Ninety-nine percent of all deaths in children under age five occur in resource-limited regions, in part because these settings lack the ability to safely oxygenate and ventilate their patients [28- 35]. In this study, we examined a prototype ultra-low-cost Venturi blender that does not use electricity, and we tested the effect of geometrical variables and process parameters on delivered FiO2
Materials and Methods
Ambient air-oxygen blender
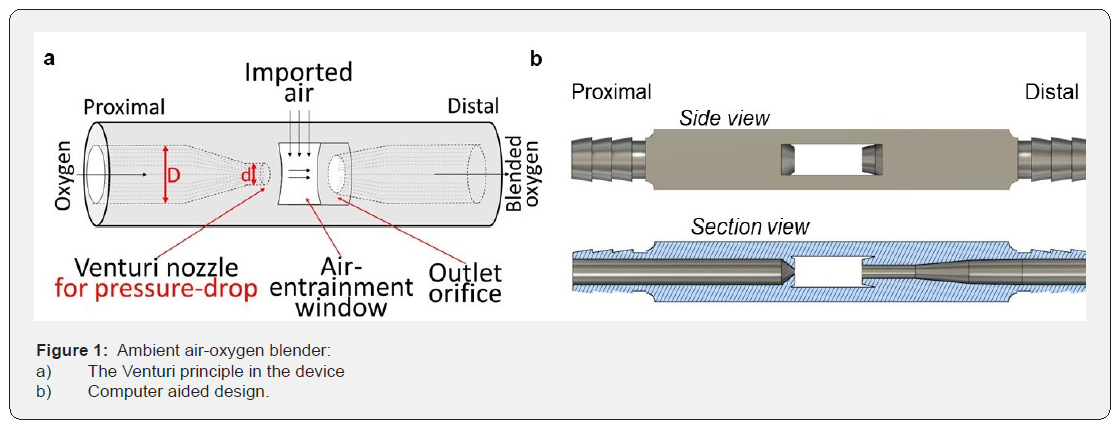
Device design: We designed an ambient air-oxygen blender where oxygen travels from a tank to a small-diameter Venturi nozzle on one side of the device (Figure 1a). Oxygen ejects from the Venturi nozzle after it is accelerated through a cylinder that rapidly shrinks from diameter D to d (Figure 1a). The progressive decrease in diameter of the channel increases the velocity of oxygen and decreases pressure in the air-entrainment chamber. The pressure differential between ambient air and the airentrainment chamber provides a driving force to draw ambient air into the air-entrainment window and mix it with the stream of pure oxygen. The ambient air–oxygen blender is designed to generate sufficient decrease in pressure to create a flow of oxygen-enriched air at a desired, fixed FiO2. A distal outlet orifice collects the gas mixture and delivers it to a neonate via inspiratory tubing. The principles of the Venturi effect are explained in the supplemental material section. We used parameters of the Venturi effect to design an ambient air-oxygen blender. Figure 1b displays the computer aided design of the ambient air–oxygen blender. The ambient air–oxygen blender was designed with a rectangular cross-section of 7mm × 9mm and a length of 73mm. The airentrainment chamber has two symmetric, rectangular-shaped air-entrainment windows. The original dimensions were fixed at
a) 0.6mm Venturi nozzle diameter
b) 2mm outlet orifice diameter,
c) 4.5mm×15mm=135mm2 cross-sectional areas of the airentrainment windows, and
d) 10mm distance between the Venturi nozzle and the outlet orifice.
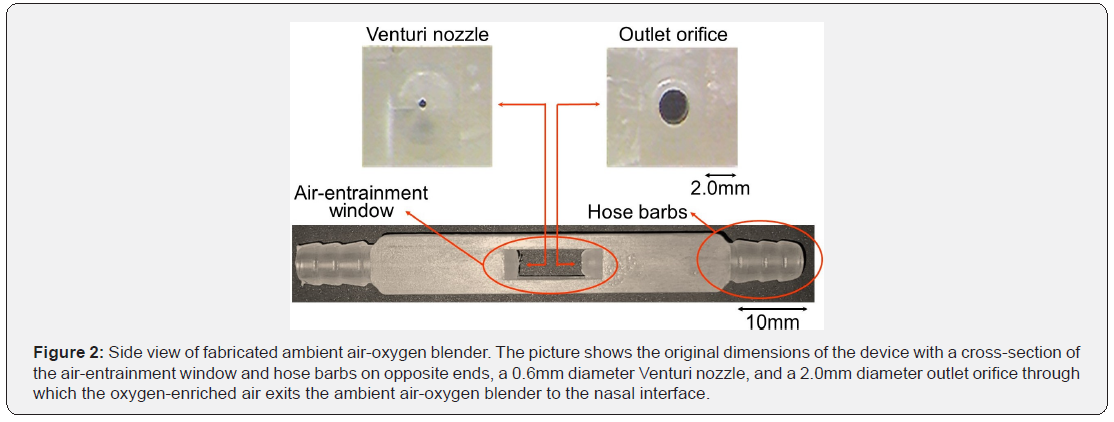
Device fabrication: Polypropylene (Mw=250,000g/mol) (Scientific Polymer Products Inc., NY) was used to fabricate the ambient air-oxygen blender. The polymer was injected into a mold at 240 °C in a one-step procedure with the use of a benchtop injection molding machine (Medium Machinery LLC, VA), and then slowly cooled to room temperature. The design of the mold is explained in the supplemental material section (Figure S2). Three grams of polymer were required per ambient air-oxygen blender. Figure 2 demonstrates the side view of an injection molded ambient air-oxygen blender.
FiO2 evaluation
For evaluation of FiO2, cylinders containing medical USP grade, pressurized oxygen (size E aluminum cylinder, CGA-VIPR with walk- O2-bout regulator and DISS integrated valve, Airgas, MA) delivered pure oxygen to the blenders (n=6 for all experiments). A Handi+oxygen analyzer (Maxtec, UT) was used to measure FiO2. ¼”(=6.35mm) ID polyethylene tubing attached blenders to the analyzer’s barded adaptor. This standard diameter oxygen tubing was used to simplify the experiment due to the large variety of tubing styles and user interfaces used for oxygen therapy, CPAP, and other neonatal therapies that may use this blender. Gas exited the system through two symmetric 3 mm diameter holes in the barbed adapter (equal to one 4.24mm D circle). The holes were similar in size to the prongs found in high flow oxygen therapy and many CPAP devices.
Evaluation of the effect of geometric variables on FiO2: Four geometric variables were evaluated:
a) Venturi nozzle diameter,
b) Outlet orifice diameter,
c) Cross-sectional area of the air-entrainment windows, and
d) Distance between the Venturi nozzle and the outlet orifice
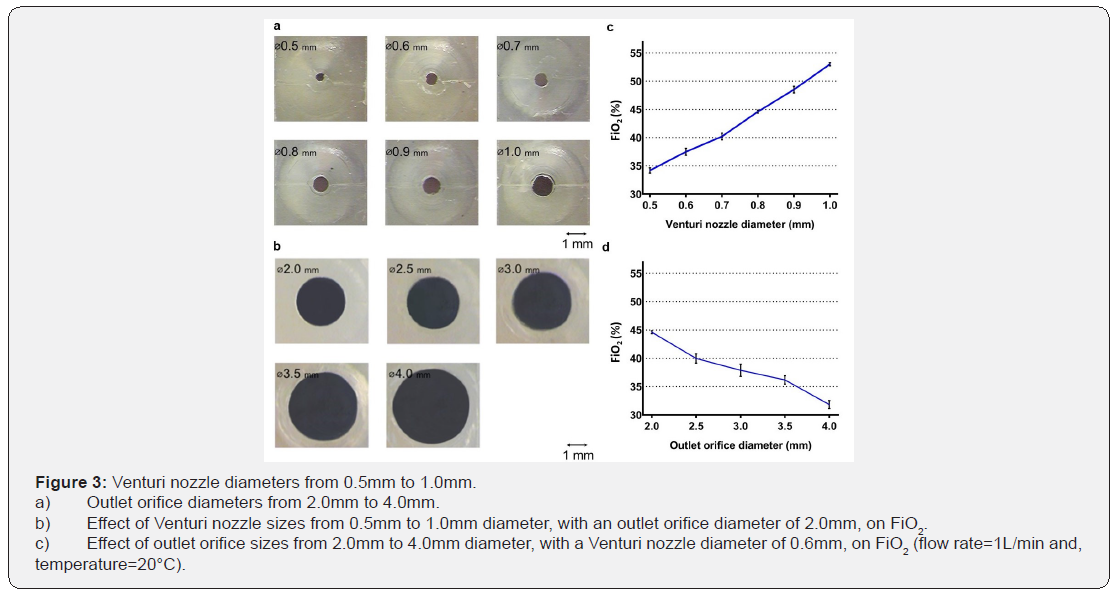
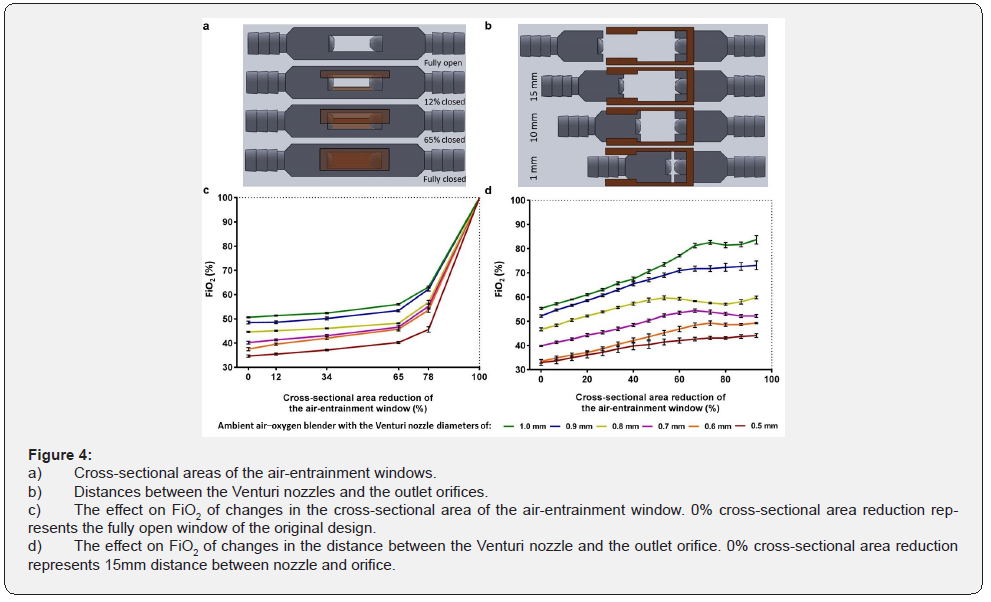
All investigations of the effect of geometric variables on FiO2 were performed at an oxygen flow rate of 1L/min. When one variable was evaluated, all other dimensions were fixed at the original values described previously. The effect of Venturi nozzle diameter on FiO2 was evaluated by fabricating ambient airoxygen blenders with Venturi nozzle diameters of 0.5mm, 0.6mm, 0.7mm, 0.8mm, 0.9mm, and 1.0mm. Multiple side cores of the negative mold were designed and fabricated to produce ambient air-oxygen blenders with Venturi nozzles with these diameters. Additionally, the effect of the outlet orifice diameter on FiO2 was evaluated by drilling separate blender outlet orifices to diameters of 2.5mm, 3.0mm, 3.5mm, and 4.0mm. The cross-sectional area of the air-entrainment window (originally 135mm2) was altered to determine the effects on FiO2. Both air-entrainment windows were simultaneously and systematically closed along a direction orthogonal to the jet flow to 78%, 65%, 34%, 12%, and 0% of their original size. The impact of these changes on FiO2 were examined. Additionally, the distances between the Venturi nozzles and the outlet orifices (originally 10mm) were altered to determine the effect on FiO2. The body of the ambient air–oxygen blender was sectioned into two parts. The parts were aligned in a track that allowed the distances between the Venturi nozzles and the outlet orifices to vary. The Venturi nozzle-outlet orifice distances were adjusted by 1mm increments from 1mm to 15mm.
The effect of process parameters on FiO2: The effect of variable oxygen upstream flow rates and air temperatures on FiO2 was examined over a flow-rate range of 0.25 to 15L/min and a temperature range of 20 to 50 °C. The tubing and the ambient air–oxygen blenders were placed inside a Fisher Scientific Isotemp oven (Pittsburgh, PA) to modify and test the ambient-air temperature. The oxygen tank was safely placed outside the oven. The original blender dimensions were used for these experiments.
Statistical analysis
Descriptive statistics (mean ± standard deviation, and standard error of mean for error bars) were used to describe quantitative variables. The relationships between variables were assessed with the use of the paired T-test and paired analysis of variance. Any probability value (p-value) less than 0.05 was considered statistically significant.
Results
The effect of ambient air–oxygen blender geometry one FiO2
The relationships between Venturi nozzle diameters, outlet orifice diameters, cross-sectional areas of the air-entrainment windows, and distances between the Venturi nozzles and the outlet orifices, on delivered FiO2 were evaluated individually by fabricating and testing ambient air–oxygen blenders with various geometric designs.
The effects of the Venturi nozzle and outlet orifice diameters: Ambient air-oxygen blenders were fabricated with Venturi nozzle diameters ranging from 0.5mm to 1.0mm and an outlet orifice diameter of 2.0mm to evaluate the effect on FiO2 (Figure 3a). FiO2 percentages increased linearly from 34.2±1.2% to 53.0±0.7% as Venturi nozzle diameters increased from 0.5mm to 1.0mm (Figure 3b). This confirmed that the ambient air–oxygen blender successfully drew air into the air-entrainment chamber to create a reduced concentration of oxygen, dependent on the nozzle diameter. Ambient air-oxygen blenders with outlet orifice diameters ranging from 2.0mm to 4.0mm and a fixed Venturi nozzle diameter of 0.6 mm were fabricated to evaluate the effect on FiO2 (Figure 3c). Increasing the diameters of the outlet orifices from 2.0mm to 4.0mm decreased FiO2 from 44.6±0.7% to 31.8±1.7% monotonically (Figure 3d).
The effect of window size and the distance between nozzle and orifice: The effect of changes in the cross-sectional areas of the air-entrainment windows and the distances between the Venturi nozzles and the outlet orifices on the exiting FiO2 was studied (Figure 4a&4b). Decreases in the cross-sectional areas of the air-entrainment windows increased the FiO2 exponentially (Figure 4c). Complete closure of the air-entrainment window on both sides produced an FiO2 of 100%. A decrease in distance between the Venturi nozzles and outlet orifices increased the FiO2 linearly (Figure 4d). The 15-mm and 0-mm Venturi nozzleoutlet orifice distances were taken to be fully open (0% decrease in the cross-sectional area) and fully closed (100% decrease in the cross-sectional area), respectively. FiO2 increased from 33.0±3.0% to 44.1±1.9% when the distance between the Venturi nozzle and the outlet orifice decreased from 15mm to 1mm, with a Venturi nozzle diameter of 0.5mm and an orifice diameter of 2.0mm. FiO2 increased from 55.3±1.1% to 83.7±4.4% as the distance between the Venturi nozzle and the outlet orifice decreased from 15mm to 1mm, for a Venturi nozzle diameter of 1.0mm and an orifice diameter of 2.0mm (Figure 4d).
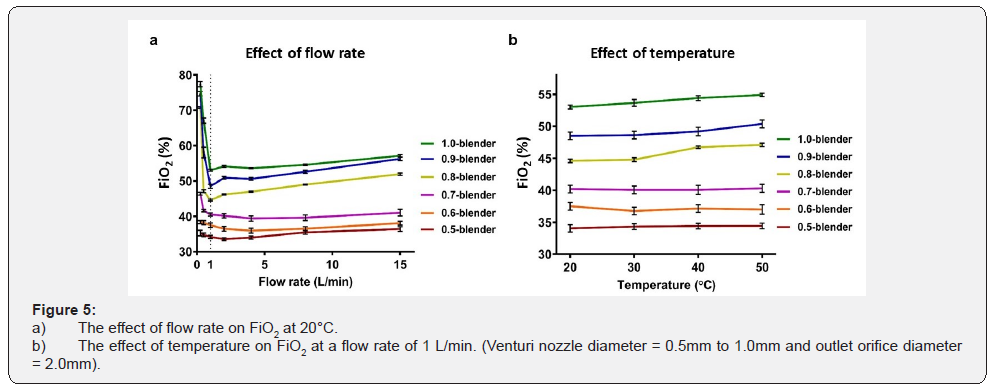
The effect of process variables
For Venturi nozzle diameters between 0.7mm and 1.0mm and a fixed orifice diameter of 2.0mm, the FiO2 varied considerably for oxygen upstream flow rates ranging from 0.25 to 1L/min (Figure 5a). However, with Venturi nozzle diameters of 0.5mm and 0.6mm with a fixed orifice diameter of 2.0mm, the FiO2 did not change with increases in oxygen upstream flow rates (P value = 0.14 and 0.15, respectively). The FiO2 increased slightly with increases in upstream flow rates from 1 L/min to 15 L/min for all Venturi nozzle diameters (Figure 5a). The FiO2 increased with temperature when the Venturi nozzle diameters were greater than 0.7mm and the orifice diameter was fixed at 2.0mm (P value <0.001, <0.001, and =0.022 for Venturi nozzle diameters of 0.8mm, 0.9mm, and 1.0mm, respectively) (Figure 5b). The largest effect of temperature on FiO2 was observed when the Venturi nozzle diameter was 0.8mm and the orifice diameter was 2.0mm. In this case, the FiO2 increased from 44.6±0.6 to 47.1±0.7 when the temperature increased from 20 to 50 °C.
Discussion
Preterm newborns and neonates with respiratory distress often need supplemental oxygen [1,36]. In high-income settings commercial oxygen blending devices are used to optimize oxygen delivery and prevent hyperoxia in these newborns. Limited designs for neonates, the need for uninterrupted electricity and compressed air when non-invasive ventilation is necessary, and high costs make these devices inaccessible across most resource-limited settings [37-42]. In this study we demonstrated that ambient air-oxygen Venturi blenders can be designed and fabricated that provide fixed oxygen concentrations in the range of FiO2 necessary for quality care of neonates in respiratory distress. By controlling the diameters of the Venturi nozzle and orifice, the cross-sectional area of the air-entrainment window, and the distance between the Venturi nozzle and the outlet orifice, we were able to create a range of FiO2 from 34% to 100%. As predicted by the Venturi principle (equation 3 and Figure S1 in the supplemental material) we found that increases in Venturi nozzle diameters (Figure 3c) decreased pressure-drops in the airentrainment chamber, which consequently lessened the draw of ambient air and thereby increased FiO2. In contrast, increasing orifice diameters drew in higher volumes of ambient air and decreased FiO2 (Figure 3d), while decreases in the cross-sectional area of the air-entrainment window allowed less air into the airentrainment chamber and consequently increased FiO2 (Figure 4a and c). Finally, increases in distances between the Venturi nozzles and outlet orifices increased the cross-sectional area of the air-entrainment windows (Figure 4b) and thereby increased the amount of available ambient air, resulting in decreases in FiO2 (Figure 4d). Control and optimization of the individual features and interdependent relationships of these four geometric parameters allowed for fabrication of a Venturi ambient airoxygen blender with tolerances necessary for care of premature newborns and infants with respiratory distress. Since treatments such as low flow oxygen therapy and CPAP require different flow rates of mixed gas, and neonates on CPAP and other sealed oxygen delivery systems require different flow rates to meet their minute ventilation requirements, we evaluated the effect of varying upstream oxygen flow rates on inspired (downstream) FiO2. Consistent with the Venturi principle (equation 3 and Figure S1), we found that when Venturi nozzle diameters were greater than 0.6mm with orifice diameters of 2.0mm, and oxygen flow rates less than or equal to 1L/min (Figure 5a), increases in upstream flow rates increased pressure-drops, drew in more ambient air, and caused decreases in downstream FiO2. However, it was surprising to discover that with these same geometric parameters, once upstream flow rates surpassed 1L/min this relationship reversed and FiO2 increased slightly with increases in oxygen flow rates. The findings from subsequent experiments that downstream FiO2 minimally changed with increases in upstream flow rates are critical for informing the design of further blenders. It appears that greater pressure-drops with this geometry do not facilitate increased volumes of air to mix with the oxygen stream above a “saturation” value (represented by the dotted line in Figure 5a). This discovery was an important additional factor to consider in blender design so that delivered FiO2 is not significantly affected by variations in flow rates
To account for various climates around the world where premature newborns are cared for, we additionally examined the effect of temperature changes on FiO2 downstream from the blender. We discovered that changes in ambient air temperatures between 20 and 50°C did not affect delivered FiO2 across varying flowrates. Strengths of our novel Venturi blender include fabrication from an inexpensive thermoplastic polymer via injection molding, low-weight and handheld, no use of electricity or compressed air, and essentially maintenance free. These qualities increase the potential for use across resource-poor settings worldwide. The primary limitation of our study is that flow rates of mixed gas downstream of the blender were not measured, and they may be affected by FiO2, upstream flow rates, oxygen loss through the entrainment window, and resistance of the downstream device. Further research is needed to better understand the effects of blender geometry on FiO2 and the efficiencies of different designs in capturing blended gas mixtures. While this study focused on addressing the limitations of existing blenders using a different design and evaluating the effect of geometric parameters on provision of a wide range of FiO2, future studies are required to comprehensively evaluate more variables that impact blender performance, such as resistance and oxygen loss. The authors are currently optimizing an ambient air–oxygen blender for a variety of downstream devices (low/ high flow cannula, CPAP setups, etc.) with comprehensive measurements of these variables in separate studies. It is likely that unique blenders will need to be designed for individual downstream apparatus specifications.
Conclusion
This study provides evidence that ultra-low-cost, Venturi ambient air-oxygen blenders can be manufactured and deliver a wide range of FiO2 without the need of electricity, motors, or compressed air, for use with neonatal therapies requiring supplemental oxygen. These properties, together with the low fabrication cost, make our ambient air-oxygen blender attractive for use in resource-poor settings.
Acknowledgement
We thank Katelyn Chan, Mike Eisenstein, Gene Saxon, and Alice Won, MD for their contributions.
Supplemental Material
Materials and Methods
Venturi principal in ambient air–oxygen blender: The diameter of the cylindrical oxygen inlet cavity (D) and the Venturi nozzle diameter (d) are device geometric dimensions which affect the pressure-drop on the Venturi nozzle side (Figure 1a). The gas flow rate and the gas density are oxygen characteristics that affect the pressure-drop [43]. The pressure-drop between the oxygen inlet cavity and the Venturi nozzle side of the ambient air–oxygen blender can be calculated with the use of the Bernoulli equation: [44]

Where P is pressure, 1 2 2ρν the kinetic energy per unit volume, and ρ gh the potential energy per unit volume.
The relationship between flow velocity and volumetric flow rate is shown in the following equation:

Where v is the flow velocity, Q the volumetric flow rate, and D the diameter of the cylindrical cavity of the ambient air–oxygen blender inlet. Since the potential energies in both the cylindrical oxygen inlet cavity and the cylindrical Venturi nozzle are identical, the pressure-drop is related to the flow rate and the geometry of the ambient air–oxygen blender per the following equation:

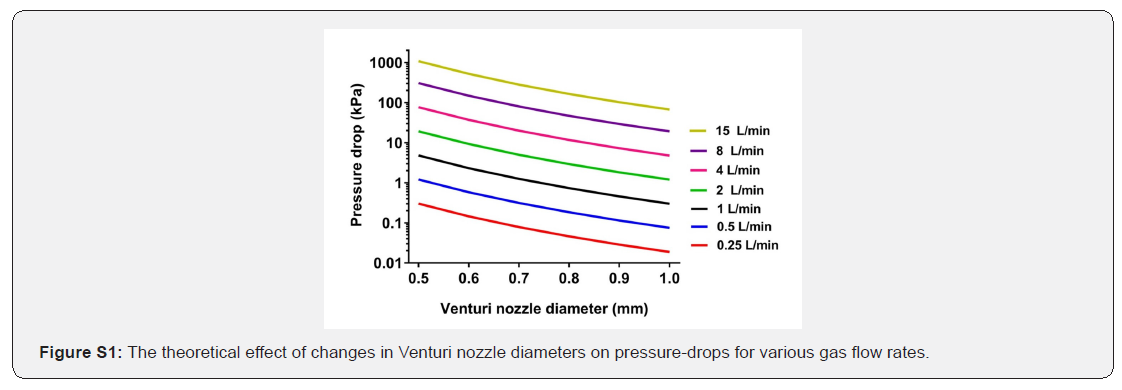
Where ΔP is the pressure-drop, ρ the density of oxygen*, Q the volumetric flow rate, D the diameter of the ambient air-oxygen blender inlet, and d the Venturi nozzle diameter. The influence of the Venturi nozzle diameter and volumetric flow rate on the changes in pressure is presented by Figure S1.
Mold design
To fabricate the blender, a four-piece negative mold was designed. The mold consisted of two cavity halves to produce the main body and the air-entrainment window, and two side sections to create the gas channels, the Venturi nozzle, and the outlet orifice. This design allows ambient air-oxygen blenders with various Venturi nozzle and outlet orifice sizes to be made simply by replacing the side pieces. The mold was fabricated by stainless steel (Figure S2).
References
- Saugstad OD, Oei JL, Lakshminrusimha S, Vento M (2019) Oxygen therapy of the newborn from molecular understanding to clinical practice. Pediatric research85(1):20-29.
- Frey B, Shann F (2003) Oxygen administration in infants. ADC Fetal and Neonatal Edition 88(2):84-88.
- Walsh M, Engle W, Laptook A, Kazzi SN, Buchter S, et al. (2005) Oxygen delivery through nasal cannulae to preterm infants: can practice be improved? Pediatrics 116(4):857-861.
- Yoder BA, Manley B, Collins C, Ives K, Kugelman A, et al. (2017) Consensus approach to nasal high-flow therapy in neonates. J Perinatol 37(7):809-813.
- Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, et al. (1999) Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics103(2):24.
- Diblasi RM (2009) Nasal continuous positive airway pressure (CPAP) for the respiratory care of the newborn infant. Respir Care54(9):1209-1235.
- Cummings JJ, Polin RA (2016) Noninvasive Respiratory Support. Pediatrics137(1).
- Saugstad OD (2005) Oxygen for newborns: how much is too much? J Perinatol 25(2):45-49.
- Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, et al. (2016) Early Cumulative Supplemental Oxygen Predicts Bronchopulmonary Dysplasia in High Risk Extremely Low Gestational Age Newborns. J Pediatr 177:97-102.
- Seiberth V, Linderkamp O (2000) Risk factors in retinopathy of prematurity. a multivariate statistical analysis. Ophthalmologica 214(2):131-135.
- Organization WH. WHO recommendations on newborn health: guidelines approved by the WHO Guidelines Review Committee
- Nishimura M (2015) High-flow nasal cannula oxygen therapy in adults. J Intensive Care 3(1):15.
- Falk M, Donaldsson S, Drevhammar T (2018) Infant CPAP for low-income countries: An experimental comparison of standard bubble CPAP and the Pumani system. PloS one 13(5):e0196683.
- Neighbour R, Eltringham R, Reynolds C, Meek J (2016) Affordable CPAP in low income countries. Anaesthesia31:63-65.
- Kubo T, Nakajima H, Shimoda R, Seo T, Kanno Y, Kondo T, et al. (2018) Noise exposure from high-flow nasal cannula oxygen therapy: a bench study on noise reduction. Respiratory care 63(3): 267-273.
- Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. (2014) Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med 190(3):282-288.
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. (2014) Every newborn: progress, priorities, and potential beyond survival. The Lancet384(9938):189-205.
- Organization WH (2015) World health statistics 2015. World Health Organization.
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. (2015) Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet385(9966):430-440.
- Organization WH (2005) The world health report 2005: Make every mother and child count.
- The lancet newborn survival series
- UNICEF (2007) The state of the world's children 2008: Child survival. Unicef.
- Morley SL (2016)Non-invasive ventilation in paediatric critical care. Paediatr Respir Rev20:24-31.
- Owen LS, Manley BJ, Davis PG, Doyle LW (2017) The evolution of modern respiratory care for preterm infants. The Lancet389(10079):1649-1659.
- Azad K, Mathews J (2016) Preventing newborn deaths due to prematurity. Best Pract Res Clin Obstet Gynaecol 36:131-144.
- Adair-Rohani H, Zukor K, Bonjour S, Wilburn S, Kuesel AC, Hebert R, et al. (2013) Limited electricity access in health facilities of sub-Saharan Africa: a systematic review of data on electricity access, sources, and reliability. Glob Health Sci Pract 1(2):249-261.
- Daga bS, Joshi H, Gunjal P, Mhatre S (2016) An Innovative Air-Oxygen Blender for Continuous Positive Airway Pressure Support in Resource-Poor Locations: A Feasibility Study. J Trop Pediatr 63(4):269-273.
- Jobe AH, Kallapur SG (2010) Long term consequences of oxygen therapy in the neonatal period. Semin Fetal Neonatal Med 15(4):230-235.
- Organization WH. Oxygen therapy for children
- Schlobohm RM, Falltrick RT, Quan SF, Katz JA (1981) Lung volumes, mechanics, and oxygenation during spontaneous positive-pressure ventilation: the advantage of CPAP over EPAP. Anesthesiology55(4):416-422.
- Saunders RA, Milner AD, Hopkin IE (1976) The effects of continuous positive airway pressure on lung mechanics and lung volumes in the neonate. Biol Neonate29(3-4):178-186.
- Katz JA, Marks JD (1985) Inspiratory work with and without continuous positive airway pressure in patients with acute respiratory failure. Anesthesiology63(6):598-607.
- McCoskey L (2008) Nursing care guidelines for prevention of nasal breakdown in neonates receiving nasal CPAP. Adv Neonatal Care 8(2):116-124.
- Fromm RE, Lechin AE, Varon J, Hirshkowitz M (1995) CPAP machine performance and altitude. Chest 108(6):1577-1580.
- Irwin Sherman T, Blackson T, Touch S, Greenspan J, Shaffer T, et al. (2003) Physiologic effects of CPAP: application and monitoring. Neonatal Netw22(6):7-16.
- Sivanandan S, Agarwal R, Sethi A (2017) Respiratory distress in term neonates in low-resource settings. Semin Fetal Neonatal Med 22(4):260-266.
- Duke T (2014) CPAP: a guide for clinicians in developing countries. Paediatr Int Child Health34(1):3-11.
- Jeeva Sankar M, Sankar J, Agarwal R, Paul VK, Deorari AK, et al. (2008) Protocol for administering continuous positive airway pressure in neonates. Indian J Pediatr75(5):471-478.
- Lissauer T, Duke T, Mellor K, Molyneux L (2017) Nasal CPAP for neonatal respiratory support in low and middle-income countries. Arch Dis Child Fetal Neonatal Ed 102(3):194-196.
- Gupta S, Donn SM (2016) Continuous positive airway pressure: to bubble or not to bubble? Clin Perinatol 43(4):647-659.
- Jensen EA, Chaudhary A, Bhutta ZA, Kirpalani H (2016) Non-invasive respiratory support for infants in low- and middle-income countries. Semin Fetal Neonatal Med 21(3):181-188.
- Dewez JE, Broek Nvd (2017) Continuous positive airway pressure (CPAP) to treat respiratory distress in newborns in low- and middle-income countries. Trop Doct47(1):19-22.
- Kala A, Mittal S, Choudhary M (2015) Characteristics of flow meters with sediment laden flow-a review.
- Pnueli D, Gutfinger C (1997) Fluid mechanics. Cambridge University Press.
- Baxter GP, Starkweather HW (1924) The density of oxygen. Proc Natl Acad Sci USA10(12):479-483.






























