Postnatal Follow-Up of Newborns with Prenatal Diagnosis of Isolated HydronephrosisM
Sylvia Foldi1, Rima Krawitsky2, Renata Yakubov3, Erez Nadir1, Amit Hochberg1 and Michael Feldman1
1 Department of Neonatology, Hillel Yaffe Medical Center, Israel
2 Department of Radiology, Hillel Yaffe Medical Center, Israel
3 Department of Pediatrics, Hillel Yaffe Medical Center, Israel
Submission: February 06, 2016; Published: March 28, 2017
*Corresponding author: Sylvia Foldi, Department of Neonatology, Hillel Yaffe Medical Center, P.O. Box 169, Hadera , 38100 Israel.
How to cite this article: Foldi S, Krawitsky R, Yakubov R, Nadir E, Hochberg A, Feldman M. Postnatal Follow-Up of Newborns with Prenatal Diagnosis of Isolated Hydronephrosis. Acad J Ped Neonatol. 2017; 4(1): 555686. DOI: 10.19080/AJPN.2017.04.555686
Abstract
Objectives: To examine the correlation between the degree of renal pelvic dilatation, and the presence of bilateral dilatation detected within the first 2-5 days of life and the outcome. To consider decreasing the postnatal examinations.
Study design: Term newborns with antenatal hydronephrosis who were born in our institution between1/1/2011and 12/31/2012 were included. They underwent one ultrasound evaluation at age 2-5 days and a second one at age 4-6 weeks, after which they were referred to our nephrologist.
Results: A total of 143 were included. Of those 132 babies completed both examinations, the first ultrasound detected bilateral hydronephrosis in 33 and the second ultrasound detected bilateral hydronephrosis in 27. Eight of the 10 infants with ureteropelvic junction obstruction had severe RPD. Six needed surgical intervention.
Conclusion: An ultrasound performed within the first 2-5 days of life can be used cautiously as a predictor of the need for further investigation; it can be useful for parental counseling as well. Children with persistent mild to moderate RPD probably do not need VCUG.
Keywords: Hydronephrosis; Newborn; Renal pelvic dilatation
Abbrevations: AB: Antibiotic treatment; ANH: Antenatal Hydronephrosis; MAG3: Diuretic renography with Tcm99MAG3; OP: Operation; RPD: Renal Pelvic Dilatation; UPJ: Ureteropelvic Junction; US: Ultrasound; UTI: Urinary Tract Infection; VCUG: Voiding Cystourethrography; VD: Vesicular Diverticulosis; VUR: Vesicoureteral Reflux
Introduction
Fetal hydronephrosis is a common finding on antenatal ultrasound (US) occurring in 1-5% of pregnancies worldwide. It is more frequent during the second or third trimester; rarely can be detected as early as the -12th-14th week of gestational age [1,2]. In 40-88% of the cases renal pelvic dilatation (RPD) is transient, resolving after the age of 18 months [3,4]. Urinary tract obstruction and vesicoureteral reflux (VUR) are recognized as main causes of severe RPD. Newborns with antenatal hydronephrosis (ANH) are at higher risk of VUR compared to the general population, with an incidence of 8.6% and 1% respectively [5]. ANH requires follow-up and sometimes antibiotic prophylaxis, some of the ANH needs invasive procedures and exposes the infant to radiation and anesthesia. The postnatal follow-up is related to the cause and severity of the ANH. Unlike infants with moderate to severe RPD, those with mild RPD mostly need only clinical surveillance [6], in order to identify babies with significant underlying pathology. Riccabona et al. recommended that babies with isolated ANH have at least two postnatal ultrasounds [7], the first more than two days postpartum, due to extracellular fluid shifts, and the second 4-6 weeks. Some of the infants with abnormal results should have a further series of US and invasive examinations such as voiding cystourethrography (VCUG) or Tcm99MAG3 renography [2,7,8]. Antibiotic prophylaxis in newborns with mild to moderate hydronephrosis is currently not recommended.
To circumvent compliance problems, we performed the initial US before discharge from hospital. In this study we aimed to determine the outcome of RPD detected by US between the 2nd -5th day of life. We also examined whether the mild RPD found in otherwise healthy babies could simply be a variant of normal anatomy and if so, whether fewer postnatal examinations are sufficient.
Subjects and Methods
This study is a single-institution retrospective chart-review of 143 infants diagnosed with prenatal hydronephrosis and followed postnatally over a 2-year period, January 1st 2011 and December 31st 2012 during which 8370 live births were treated in our hospital. Ethical approval was obtained from the IRB. Term babies (≥36+6 weeks) who were diagnosed by prenatal US as having isolated ANH were included. In our population there were only few mothers with complete antenatal documentation regarding the degree, the laterality and the exact gestational age at the time of the finding. We decided to omit the antenatal measurements since most of our patients arrived without the complete data, it was impossible to set a cut-off of antenatal RPD. Subjects with additional congenital anomalies or genetic syndromes were excluded. Newborns with double collecting system, single kidney or horseshoe kidney, multicystic kidney disease were also excluded.
We defined mild hydronephrosis as RPD 4-9.9mm, moderate hydronephrosis as RPD 10-14.9mm and severe hydronephrosis as RPD>15mm. Several systems of grading hydronephrosis are known [9]. We used the system of Mami et al. [4] that was consistent with the recommendations of our nephrologist.
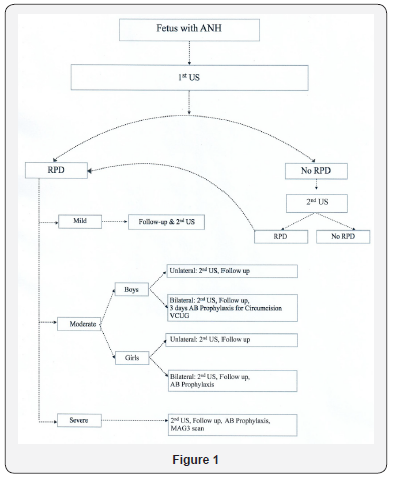
The first US was performed when the infant was 2-5 days of age before the discharge from hospital. All the results were read by the same radiologist. The babies were recommended to undergo a second US at the age of 4-6 weeks in their community health centers, after which they returned for clinical followup and remained under surveillance up to 18-24 months. US results, medical interventions, treatments and diagnoses were documented in their files (Figure 1).
Statistical analysis
Demographic data are presented in tables using the means and standard deviations (Table 1 & 2). Nominal and ordinal data are described by distribution tables. Student’s t-test and the ANOVA test were used to compare means and assess significant differences. Nominal variables were analyzed by the chi-square test to detect differences between frequencies in case of gender. Statistical significance was set at a P- value <0.05. All calculations were done using IBM SPSS software version 20.
Results
Patient population
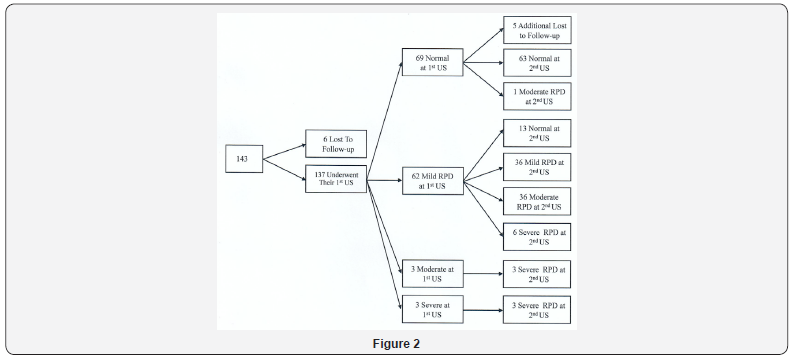
During the two year period of our study 143 (1.7%) term neonates were diagnosed with some degree of ANH, of whom 137 completed one and 132 completed both US studies (Figure 2). In most of the cases we had no exact data about the degree of the ANH. Similarly the exact gestational age of the finding of the ANH or the laterality was not well documented.
There were 105 (73%) males and 38 (27%) females. All males underwent ritual circumcision up to the age of one month.
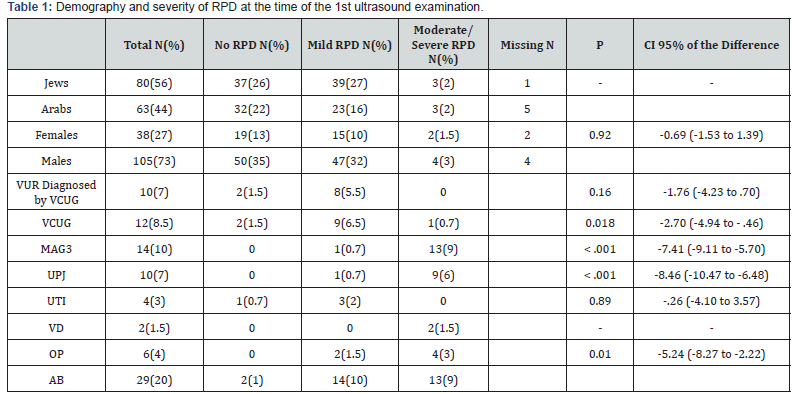
There was no correlation between male gender and the severity of RPD: P=0.92 for the first US, P=0.85 for the second US (Table 1 & 2).
Analysis of male gender and the presence of bilateral hydronephrosis also revealed no correlation: P=0.93 for the first US and P=0.32 for the second US (Table 3).
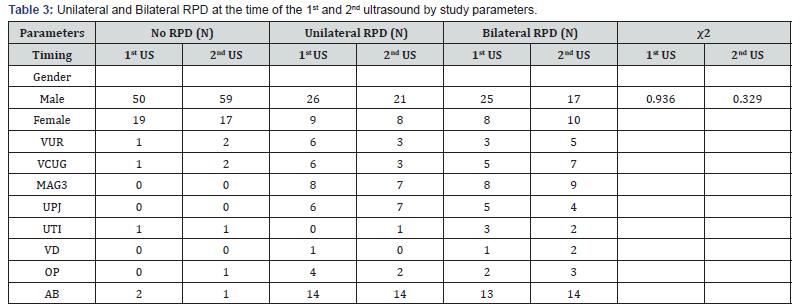
AB: Antibiotic treatment; CI: Confidence Interval; MAG3: Diuretic renography with Tcm99MAG3; OP: Operation; RPD: Renal Pelvic Dilatation; UPJ: Ureteropelvic Junction; US: Ultrasound; UTI: Urinary Tract Infection; VCUG: Voiding Cystourethrography; VD: Vesicular Diverticulosis; VUR: Vesicoureteral Reflux.
Distribution of RPD during the first examination
According to the first postnatal US 69 out of 143 (48%) newborns had no dilatation while 62 (43%) had mild, 3 (2%) had moderate and 3 (2%) had severe dilatation. One of the three children with severe dilatation had bilateral RPD and all three were referred to an early VCUG but there was no evidence of VUR. Further investigation included a diuretic renography that detected UPJ obstruction in all three babies. Their follow- up revealed further dilatation of the renal pelvis. One of them had surgical repair and the RPD of the other two stabilized and regressed by the age of 18 months. Thirty-three (23%) infants were found to have bilateral hydronephrosis (Table 4).
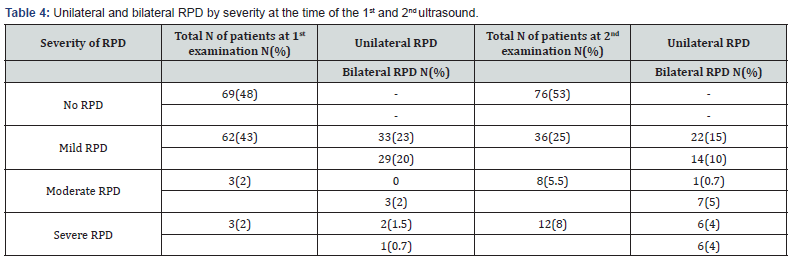
RPD indicates renal pelvic dilatation.
Distribution of RPD during the second examination
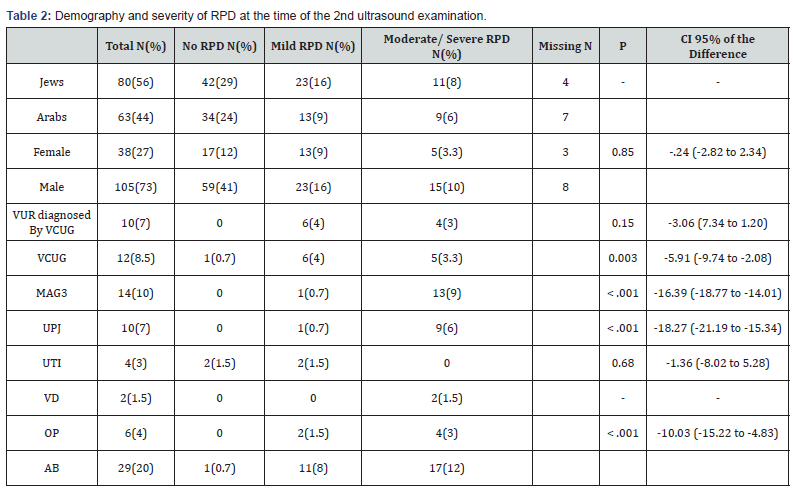
Second US was normal in 76 infants (53%). Of them 63 infants remained with normal findings, and one was diagnosed as having moderate RPD at the age of 4-6 weeks. Five infants from the group with the normal examination never completed their second US, their parents were not interested in further follow- up. At the time of the second US, 36 newborns (25%) remained with mild dilatation. All three babies with moderate RPD on the first US progressed to severe dilatation. A total of 17 babies had worsening ultrasound test (3 with previously moderate, 13 with previously mild, and 1 baby with no RPD had further dilatation) (Figure 2). Eight newborns (5%) had moderate RPD, 12(8%) had severe dilatation and 27 infants (19%) had bilateral ANH (Table 4).
Vesicular diverticulosis
Two (twin brothers) of the 12 babies with severe dilatation were diagnosed with severe bilateral hydronephrosis with the rare entity of vesicular diverticulosis in the background. Moderate bilateral RPD had been detected on the first US without detection of the diverticulosis; they received a 3-day course of prophylactic antibiotic treatment around the date of circumcision. At the age of two weeks they presented with worsening hydronephrosis. The giant vesicular diverticulae were diagnosed by MRI. They underwent emergency ureterostomy.
Uretheropelvic Junction (UPJ) stenosis
UPJ obstruction was defined by nuclear radiology of diuretic renography. Ten babies (7%) had UPJ obstruction. Severe dilatation had already been observed in three of them in their first US. The RPD worsened from moderate to severe in six infants, while one infant with mild RPD, bilateral UPJ obstruction and recurrent UTI remained stable. One infant who had moderate bilateral RPD at his first and severe bilateral RPD at his second US had no underlying anatomical malformation by the end of the 18 months follow-up.
The presence of UPJ stenosis was strongly correlated to the severity of the RPD according to both US examinations (P<0.001) (Table 1 & 2). Of 10 infants with UPJ stenosis three (30%) had bilateral severe RPD.
Vesicoureteral reflux
Ten babies (7%) had VUR diagnosed by VCUG and all of them were detected with mild or moderate RPD. Their VUR improved or resolved in all ten by the age of 18 months. Three of those ten babies with mild RPD and VUR had bilateral persistent hydronephrosis. One of the babies that had mild and persistent bilateral RPD also had VUR grade V with recurrent episodes of UTI. She needed a surgical intervention (Deflux procedure) around the age of 6 months. Additional 15 babies had mild hydronephrosis that was diagnosed by renal US and were not referred to VCUG. These babies had mild but persistent RPD that resolved during the follow-up without evidence of infection, no prophylactic antibiotic treatment was needed.
According to these results the presence of VUR did not correlate with the grade and severity of RPD in either the first or the second US study (P=0.16 and P=0.15, respectively). Five of the ten babies with VUR had bilateral RPD.
Urinary tract infection
Four infants (3%) had UTI (ascertained by the review of their medical records): one had normal results in both examinations and, the other three had a mild bilateral RPD that resolved during their follow-up. One of the four had VUR. There was no correlation between the occurrence of UTI and the grade of RPD in the first and second US examinations (P=0.89 and P=0.68, respectively) (Table 1 & 2).
Further Investigation
Fourteen infants (10%) underwent diuretic renography with Tc99mMAG3. Eight (57%) had bilateral hydronephrosis and 10 (71%) had UPJ stenosis. Of 10 infants with UPJ stenosis four (40%) had bilateral RPD and all of them had severe bilateral dilatation (Table 5).
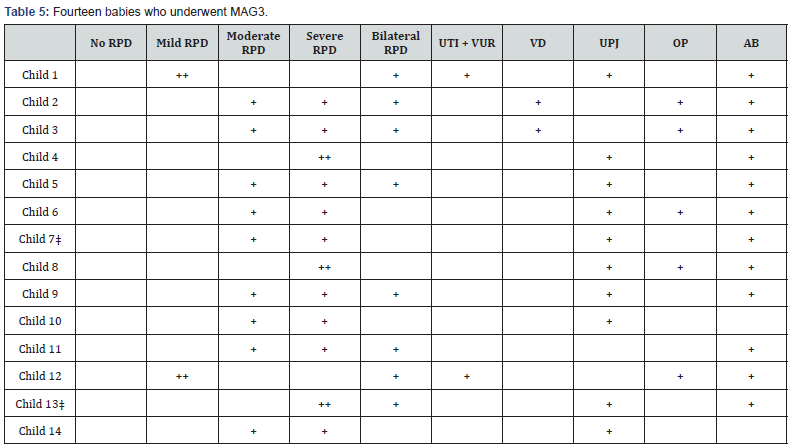
AB: Antibiotic treatment; CI: Confidence Interval; MAG3: Diuretic renography with Tcm99MAG3; OP: Operation; RPD: Renal Pelvic Dilatation; UPJ: Ureteropelvic Junction; US: Ultrasound; UTI: Urinary Tract Infection; VCUG: Voiding Cystourethrography; VD: Vesicular Diverticulosis; VUR: Vesicoureteral Reflux
‡indicates female infants
Infants 1 and 12 had mild RPD in both US examinations
Infants 4, 8 and 13 had severe RPD in both US examinations
All the other infants had moderate RPD in their 1st and severe RPD in their 2nd US examinations
Infants 10 and 14 had prophylactic AB treatment only for 3 days around the circumcision.
Twelve infants (8.5%) were referred to VCUG. VCUG was recommended for boys with bilateral hydronephrosis to rule out posterior urethral valve and for everyone after the first episode of UTI. Seven of the 12 infants (58%) who underwent VCUG had bilateral RPD. Ten (83%) infants of those 12 had VUR diagnosed by VCUG. There were 15 children who were not referred to VCUG but their VUR was diagnosed by US by visualizing the calyceal dilatation and good corticomedullary definition during their radiological follow-up. They did not need prophylactic antibiotics and no further examination, their RPD and resolved by the age of 2 years.
A total of 29 (20%) babies were started on antibiotic prophylaxis during their follow-up according to their medical records. Twenty-six had their antibiotics before diuretic renography or before VCUG. The remaining 3 infants were started on a short course of prophylaxis for three days around their circumcision: their first renal US revealed a moderate bilateral RPD.
Altogether six infants (4%) had surgical intervention (two with mild RPD and 4 with moderate to severe RPD). Three of the six had bilateral ANH. There was also a correlation between the decision to perform surgical repair and the severity of RPD: P=0.01 with the first US and P<0.001 with the second US. Criteria for surgical intervention were 1) RPD>30mm; 2) RPD>20 mm with calyceal dilatation; 3) renal function<30%; 4) worsening renal function; 5) worsening hydronephrosis; 6) symptoms of recurrent UTI; 7) renal obstruction.
Discussion
Most infants with prenatal isolated RPD have no clinically relevant findings and symptoms during their follow-up in infancy. The ability to predict the postnatal course of prenatally diagnosed hydronephrosis in a specific child is important both for the parents and for the health care provider. Previous studies reported that mild ANH detected before 30 weeks gestation resolves or stabilizes during follow-up in more than 70% of cases, suggesting that mild RPD might be a relatively benign condition [9].
We investigated whether the degree of RPD as measured during the first week of life and again at the age of 4-6 weeks can be helpful in predicting the postnatal course in terms of underlying renal disease, testing and treatment.
The exact values of the ANH were not available to us mostly because of lack of documentation. We decided to rely on the first postnatal US that was performed between the 2nd and 5th postnatal days. The decision to perform the first US before hospital discharge was planned to circumvent problems of compliance [6].
The prevalence of hydronephrosis was 1.7%, similar to the values reported in the literature [2]. We found a male predominance in our population of infants with ANH: male gender had previously been described as a risk factor for postnatal hydronephrosis [10,11]. We did not find significant difference between male and female babies in our study population regarding UPJ obstruction, VUR and UTI. There was no gender difference in prophylactic antibiotic treatment or invasive procedures (i.e. VCUG, Tc99mMAG3 and surgical intervention).
Our assessment revealed that 7% of the studied infants had VUR, a value that is similar to the 9% described by others, [2] although there are studies that describe 15% [10] or even as many as 38% [12,13]. Similarly to previous reports the degree of VUR did not appear to correlate with the degree of the RPD [14]. None of our study children with severe hydronephrosis had coexisting VUR. The entity was rather detected in infants with mild to moderate hydronephrosis. The absence of hydronephrosis, however, does not rule out the presence of VUR [15]. VUR had been considered the major risk factor for UTI and renal scarring. It was also shown however, that UTI and renal sequelae can occur without VUR and that UTI does not always cause renal scarring. On the other hand, the incidence of renal scarring is significantly higher after UTI in patients with VUR [6]. There are descriptions that children with moderate to severe antenatal RPD were at significant risk of UTI [16]. In our study three of the four infants with UTI had mild bilateral hydronephrosis, but the small numbers do not allow to define correlation between UTI and bilateral RPD. Several authors consider the use of neonatal US in screening for VUR as being controversial. It probably cannot replace VCUG in cases with high risk of VUR because it detects 25%-45% of those patients [12]. Several authorities recommended that postnatal US should include not only the measurement of RPD but also measurements of calyceal and ureteric dilatation, as well as signs of dysplasia in order to select the infants who should undergo VCUG [17-21]. VCUG may be unnecessary in infants with isolated ANH who had two postnatal US examinations with a RPD <7mm, good corticomedullary definition, mild calyceal dilatation [16]. The recommendations of our nephrologist were similar to these guidelines, these infants had 2-3 additional sonographic examinations during their follow-up without VCUG (fifteen infants).
We detected UPJ obstruction in nearly 7% of the infants, somewhat lower than the expected 11-12% in this population [2]. We could clearly demonstrate that the risk of UPJ obstruction is higher with more extensive RPD. Most of our babies who had severe dilatation were later diagnosed as having UPJ obstruction (10 out of 12 infants). Furthermore four infants with UPJ stenosis also had bilateral RPD.
The results of the renal US performed during the first 2-5 days of life can be used for parental counseling in terms of invasive testing, especially renography. It has to be acknowledged that the findings that more children had a normal renal US at the age of 4-6 weeks than at 2-5 days of life goes against many prior studies suggesting that the early renal US may be inaccurate owing to fluid loss and fluid shifts in the neonate.
The main limitations of this study are the nature of retrospective chart review, small numbers and the lack of the prenatal renal measurements. We are aware of the fact that the data of the degree and laterality of the ANH and correct prenatal documentation of the exact gestational age of the detection would add to this study. However we tried to examine the reliability of the first postnatal US examination in study populations where the antenatal followup and the patient compliance are weaker. Our study can be useful in peripheral areas where it is difficult to perform the scheduled ultrasound examinations during pregnancy.
Our study points to a greater risk- especially for UPJ obstruction- in every newborn with moderate to severe hydronephrosis confirmed during the first week of life. Bilateral isolated RPD that is detected during the first week of life carries an increased risk for postnatal pathology as well. It is still uncertain whether mild isolated RPD can be considered as being a variant of a normal anatomy. We agree that infants who have stable moderate RPD in two consecutive US examinations will not need to undergo VCUG. In these cases probably VUR can be diagnosed by US if good corticomedullary definition and mild calyceal dilatation are present with stable RPD. Based on this in our study there were 15 children with VUR who were not referred to VCUG. An ultrasound performed during the first week of life can be used cautiously as a predictor of the need for further investigation. It can be useful for parental counseling as well however as a sole assessment it is not accurate enough.
We continue to perform the initial US examinations before discharge in cases of antenatal diagnosis of hydronephrosis, we are aware of the fact that the examination is more reliable after the second postnatal day.
Acknowledgement
We would like to thank Esther Eshkol, Iris Dahan and Izhar Ben Shlomo for their helpful input in our study.
Conflict of Interest
The authors report that there is no conflict of interest in relation to this study.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Contributorship Statement
Sylvia Foldi: Dr. Foldi conceptualized and designed the study, carried out the initial analyses, drafted the initial manuscript and approved the final manuscript as submitted.
Rima Krawitsky: Dr. Krawitsky carried out the ultrasound examinations, reviewed the manuscript and approved the manuscript as submitted.
Renata Yakubov: Dr. Yakubov carried out the clinical followup of the infants and provided the data of this manuscript; she reviewed the manuscript and approved the manuscript as submitted.
Erez Nadir, Amit Hochberg: Drs. Nadir and Hochberg supervised data collection, reviewed and revised the manuscript, and approved the manuscript as submitted.
Michael Feldman: Dr. Feldman participated in the study design, critically reviewed the manuscript, and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- Robyr R, Benachi A, Daikha-Dahmane F, Martinovich J, Dumez Y, et al. (2005) Correlation between ultrasound and anatomical findings in fetuses with lower urinary tract obstruction in the first half of pregnancy. Ultrasound Obstet Gynecol 25(5): 478-482.
- Lee RS, Cendron M, Kinnamon DD, Nguyen HT (2006) Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics 118(2): 586-593.
- Nguyen HT, Herndon CDA, Cooper C, Gatty J, Kirsch A, et al. (2010) The Society for Fetal Urology Consensus Statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol 6(3): 212- 231.
- Mami C, Paolata A, Palmara A, Marrone T, Marseglia L, et al. (2010) Outcome and management of isolated moderate renal pelvis dilatation detected at postnatal screening. Pediatr Nephrol 25(10): 2093-2097.
- (1981) Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics 67(3): 392-400.
- Coelho GM, Bouzada MCF, Pereira AK, Figueiredo BF, Leite MRS, et al. (2007) Outcome of isolated antenatal hydronephrosis: a prospective cohort study. Pediatr Nephrol 22(10): 1727-1734.
- Riccabona M, Fotter R (2004) Reorientation and future trends in paediatric uroradiology. Minutes of a symposium held at Graz, 2002 5-6 Sep. Pediatr Radiol 34(4): 295-301.
- Riccabona M, Avni FE, Blickman JG, Dacher JN, Darge K, et al. (2008) Imaging recommendations in paediatric uroradiology: infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography. Conference held at Barcelona, Spain, 2007 June. Pediatr Radiol 38(2): 138-145.
- Sidhu G, Beyene J, Rosenblum ND (2006) Outcome of isolated antenatal hydronephrosis: a systematic review and meta-analysis. Pediatr Nephrol 21(2): 218-224.
- Phan V, Traubici J, Hershenfield B, Stephens D, Rosenblum ND, Geary DF (2003) Vesicoureteral reflux in infants with isolated antenatal hydronephrosis. Pediatr Nephrol 18(12): 1224-1228
- Al-Shibli AI, Chedid F, Mirghani H, Al- Safi W, Al-Bassam MK (2009) The significance of renal pelvic dilatation as a predictor of postnatal outcome. J Matern Fetal Neonatal Med 22(9): 797-800.
- Zerin JM, Ritchey ML, Chang AC (1993) Incidental vesicoureteral reflux in neonates with antenatally detected hydronephrosis and other renal abnormalities. Radiology 187(1): 157-160.
- Tibballs JM, Bruyn RD (1996) Primary vesicoureteral reflux - how useful is postnatal ultrasound. Arch Dis Child 75(5): 444-447.
- Bassanese G, Travan L, D’Ottavio G, Monastra L, Ventura A, et al. (2013) Prenatal anteroposterior pelvic diameter cutoffs for postnatal referral for isolated pyelectasis and hydronephrosis: more is not always better. J Urol 190(5): 1858-1863.
- Wilson J (2008) Postnatal follow- up of prenatally detected hydronephrosis - how much imaging is enough? J Urol 180(1): 17-18.
- Wollenberg A, Neuhaus TJ, Willi UV, Wisser J (2005) Outcome of fetal renal pelvic dilatation diagnosed during the third trimester. Ultrasound Obstet Gynecol 25(5): 483-488.
- Lidefelt KJ, Ek S, Mihocsa L (2006) Is screening for vesicoureteral reflux mandatory in infants with antenatal renal pelvis dilatation? Acta Paediatr 95(12): 1653-1656.
- Ismaili K, Avni FE, Hall M (2002) Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvic dilation. J Pediatr 141(1): 21-24.
- Avni FE, Ayadi K, Rypens F, Hall M, Schulman CC (1997) Can careful examination of the urinary tract exclude vesicoureteric reflux in the neonate? Br J Radiol 70(838): 977-982.
- Yerkes EB, Adams MC, Pope JC, Brock JW (1999) Does every patient with prenatal hydronephrosis need voiding cystourethrography? J Urol 162(3 Pt 2): 1218-1220.
- Coplen DE, Austin PF, Yan Y, Dicke JM (2008) Correlation of prenatal and postnatal ultrasound findings with the incidence of vesicoureteral reflux in children with renal pelvic dilatation. J Urol 180(4 Suppl): 1631-1634.






























