Abstract
Phenyl acetylene (PA) is one of the by-products produced in the production of styrene from dehydrogenation of ethylbenzene. It always presents in ~ 10-200 ppm level in commercial styrene, separation of this required expansive catalytic process. More than 66% of produced styrene word wide is utilized in making polystyrenes like HIPS, GPPS and EPS. This study evaluated the effect of PA quantity on styrene polymerization process, initiated by thermal/radical initiator, especially at high temperature (>100 °C). Polystyrenes (HIPS, GPPS & EPS) are prepared in 0.8-1.0kg scale with PA content of 5-200 ppm. In each recipe, the polymerization process was carefully monitored by collecting intermediate sample for every 1h to understand the molecular weight build up, conversion and consumption of PA. In GPPS, thermal initiation process at 128oC showed decrease in molecular weight (2-4 %) and drop in conversion (5-7%) for every 50 ppm, but there was no considerable change in mechanical and thermal properties. In case of HIPS, at 50 ppm and above, the izod impact drastically reduced and same trend observed in gel content. Interestingly, the deteriorated impact properties can be got back easily just by adding 25-30% of excess initiator without compromising any other properties. In the EPS process, above 50 ppm of PA, extended reaction time of + 0.07 min / ppm was required, also oversized EPS beads increased considerably.
Keywords:Phenyl acetylene (PA); Dehydrogenation; Styrene; Radical Polymerization; Expandable polystyene (EPS); Genaral purpuse polystyrene (GPPS); High impact polystyene (HIPS)
Introduction
Styrene is one of the important industrial monomers used in production of polystyrene, latexes, rubber, elastomer, thermoplastics, thermosetting etc. Majority (>66%) of styrene has been used in polystyrene production (GPPS, HIPS & EPS) for the applications such as household articles, building construction, packaging. Styrene is produced by dehydrogenation of ethylbenzene (EB)[1-3]. Phenyl acetylene (PA) is one of the undesirable by-products formed in this process, consequently, the styrene contains EB & traces of PA always. EB can be removed easily by distillation but removal of PA required an additional catalytic treatment[4-6]. The catalytic process involves injection of high level of hydrogen gas into the styrene monomer in presence of palladium/aluminium catalyst, any stoichiometric excess of hydrogen injected can lead to conversion of styrene back to EB and causing lower conversion rate [7-8]. Significant reduction in the PA is achieved only at the expanse of styrene conversion to EB and loss in styrene production [8]. Therefore,the PA content in commercial styrene always fluctuate in 10-200 ppm, and it depends on the activity & efficiency of catalytic bed of hydrogenation reactor. Many studies reported that presence of higher PA in styrene has undesirable consequences regardless of method of polymerization, like free radical, anionic & co-ordination [1-3]. Morton Litt et al. studied the kinetics of styrene emulsion co-polymerization at 50 °C with PA in presence of free radical initiator and rationalized that chain transfer radical with PA is much stable than growing polymer radical and reinitiates slowly [9]. Jurgen et al. investigated the syndio-specific homo- and copolymerization of styrene in presence of PA as a non-polar impurity. The results showed significant decrease in the polymerization conversion with increase in PA concentration without effect on molecular weight and distribution [10]. For most of the polystyrene production plant, that follow bulk polymerization method with peroxide initiators, in general, the polymerization temperature will be in the range of 100-130 °C.
Thermally generated free radicals on styrene above 100 °C can compete with free radicals generated by peroxide initiators and can create complex kinetic mechanism. However, the effect of PA on polymerisation at high temperature and properties of final polymers have not explored much in past. In industrial point of view, it is very essential to understand effect of PA on bulk polymerization at this temperature range.
Materials and Methods
Reagents
Initiators (Luperox® 26M50) from Arkema, Polybutadiene rubber (PBR) from Petrokemya and Zinc stearate from FACI were received. Styrene, Phenyl acetylene (PA), methylenedichloride (MDC), Tetrahydrofuran (THF), Ethylbenzene (EB) Toluene and other analytical solvents were procured from Sigma-Aldrich and used as such.
Gel-permeation chromatography (GPC)
The molecular weight of intermediate samples and final polymer was measured by GPC at 40 oC (Make-Shimadzu, Class-VP). THF was used as mobile and diluent solvent, sample concentration 2 mg/ml, column specification- PLgel 5μm MIXED-C, 300 x 7.5 mm, Detector- UV @254nm using Monodispersed polystyrene standards.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) of all the sample was done with TA Instruments/DSC Q 1000 by heating the samples from room temperature to 160 oC. 3-4 mg sample was sealed in aluminium pans and heating rate always maintained at 10 oC/min in nitrogen atmosphere.
High performance liquid chromatography (HPLC)
Unreacted residual styrene monomer was detected and estimated by high performance liquid chromatography (HPLC) using Agilent HPLC-1260 series instrument. The chromatographic condition are as follows: column- Agilent Zorbax,-C18, (4.6X150mm), 5μ Mobile phase: Reservoir A -0.02% Orth phosphoric acid in Milli-Q water, Reservoir B-Methanol, Reservoir C - Acetonitrile at different gradients. Flow rate 1 ml/ min, detector-diode array detector, wave lenth-254 nm. Column temperatue-40 oC
Melt flow index (MFI)
Melt flow index was measured at 230 oC & 2.16 kg load as per the ASTM D 1238, in GOTTFERT MFI machine (model-MI-4).
Heat distortion temperature (HDT)
Heat distortion temperature was measured as per the ASTM D648, method B at 0.45 MPa load, on the CEAST machine. All the samples were annealed for 70 hrs at 70 oC prior to test.
Vicat softening temperature (VST)
Vicat softening temperature was also measured in CEAST machine for annealed samples as per ASTM D1525. All the samples were annealed for 70 hrs at 70 oC prior to test.
Mechanical property (Tensile and Izod Impact notched)
Injection moulded specimens were conditioned for 48 h at 23 oC & relative humidity at 50%. Tensile strength was measured were accordance to ASTM D638, on Zwich 2.5 RTI-UTM machine. Notched Izod impact was measured as per the ASTM D256.
Optical Properties: Lab
The actual method for WI (Whiteness index) and YI (Yellowness index) measurement of GPPS pellets is ASTM E313 (as referred in ASTM D 6290). Color spectrophotometer instrument was used in reflectance mode with Illuminant-C at 2° angle.
Lab values
This is an approximately uniform colour space based on
nonlinear expansion of tri-stimulus values and taking difference
to produce three opponent axes that approximate the percept of
lightness-darkness, redness-greenness, and yellowness-blueness.
It is produced by plotting in rectangular co-ordinates.
Instrument: Color-Eye 7000A (Greatag Macbeth)
Test Standard: ASTM D2244
Measurement Set-up: Mode - Reflectance
Illuminant - D65, Observer angle-10°
SCI (Specular Component Included)
UVI (UV Included), LAV- Large Aperture View,
LLV- Large Lens View. Calibrate the color spectrometer using blank and white calibration tile. Fill the cuvette with granules and place the Cuvette on the sample port.
PA Quantification by GC
Established a GC method for the quantification of phenyl acetylene (PA) in styrene monomer, GPPS and HIPS polymers using GC-FID (gas chromatography-Flame ionization detector) external standard quantification method. In addition, identified all the major peaks in the samples by GCMS technique. All the raw materials/monomer (Phenyl acetylene, Styrene) samples were dissolved in dichloromethane solvent and analysed. Calibrated the system using Phenyl acetylene standard between 1 to 4000 ppm using dichloromethane as diluent and quantified PA content in styrene monomer and all polymer samples. For polymer samples weighed 0.5 g in a 30 mL PP Tube, added 5mL dichloromethane and kept it for shaking for 1hr. After complete dissolution, added 5 mL of acetonitrile to precipitate the polymer. Then filtered and analysed. Injected the sample in triplicates for the repeatability of the analysis. Injected couple of blanks (only DCM and acetonitrile) prior to the analysis and ensured no external peaks are eluting in the system
GEL content analysis in HIPS sample
Weigh accurately 0.6 gram of HIPS sample into a 20 mL glass vial, add 15 mL toluene in to vial and place them on the shaker until it is dissolved. Quantitatively transfer the dissolved sample into the pre-weighed SS tubes and further add 10 mL of toluene to glass vial, dissolve to recover the left-out samples and transfer the toluene solution into SS tube. Spin the SS tubes in the refrigerated centrifuge (-15°C) for about 75 minutes at 20000 RPM. Drain off the polymer supernatant (Top Layer-Free Polystyrene). With glass rod, carefully re-slurry the samples in fresh toluene. Make sure that glass rod is thoroughly rinsed in tube. Repeat the centrifugation step. Pour off the toluene supernatant (free Polystyrene) solvent, invert on to paper towel and let stand for about 5 minutes. Place the SS tubes in a vacuum oven at 85°C with 25 min. Vacuum until it dry (Overnight dry up to 18 hours). After the tubes cool, weigh the dry gel with SS tube.
Calculate the Percent gel using following formula:

Synthesis of GPPS/HIPS in Lab-scale (0.8 kg) with
Synthesis of GPPS/HIPS in Lab-scale (0.8 kg) with different PA loading
The styrene used for synthesis of GPPS/HIPS procured from Sigma-Aldrich, the PA level was less than 5 ppm. PA added externally to styrene at the beginning of polymerization based on styrene quantity. Around 750 g of GPPS/HIPS granules was obtained from each batch in lab scale glass reactor. Each batch was processed separately to get pellets of uniform size. The complete process involved three steps like synthesis, powdering, and extrusion/palletization (Figure S1-S5).
Results and Discussion
GPPS
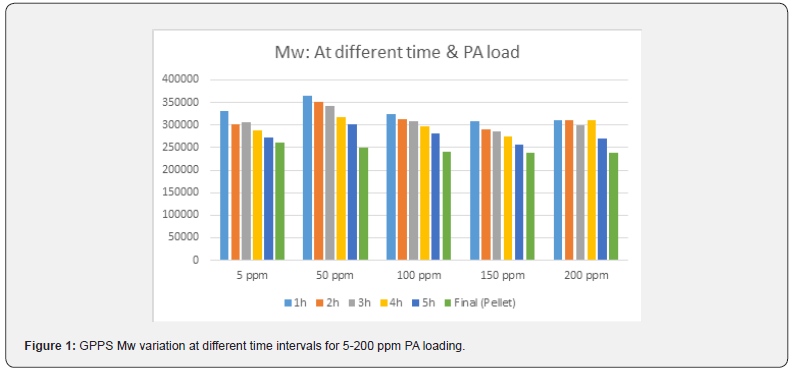
Different batches were conducted with different PA loading like 5, 50, 100, 150, 200 ppm in similar conditions. Polymerization was monitored carefully by collecting intermediate sample for every 1h and analyzed the molecular weight, conversion and PA consumption. At the end, high vacuum was applied to collect the unreacted styrene EB and PA. From the graph (Figure 1 & Table 1), it is clear that the inclination of molecular weight build-up did not change in presence of PA (5 to 200 ppm). The highest Mw achieved between 1-2 h reaction time irrespective of PA concentration. However, there is a slight decreasing trend in the final molecular weight as PA content increases. The important observation is that the unreacted styrene quantity increased in EB stream at devolve as PA content increased in styrene (Table 1). This indicates that the conversion is dropping at higher level of PA.
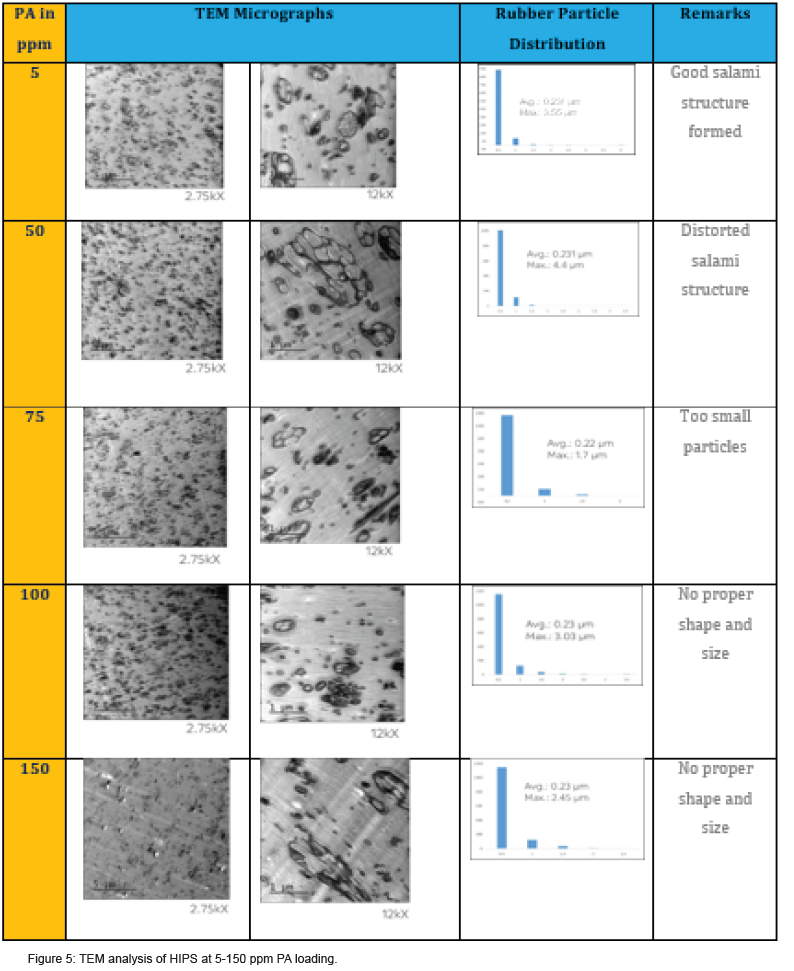
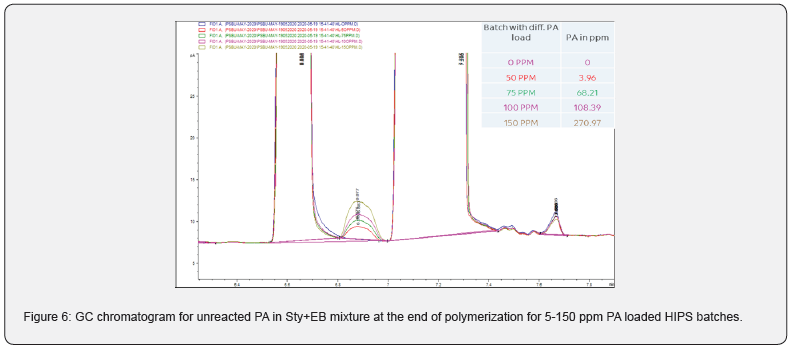
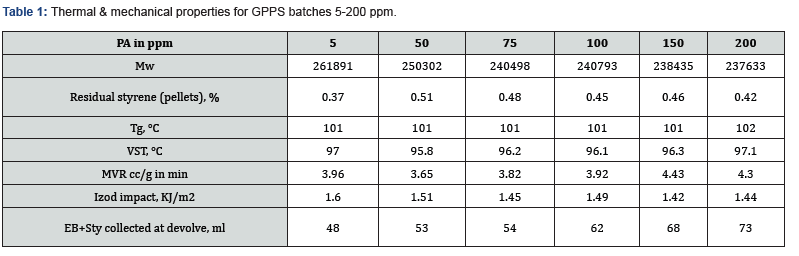
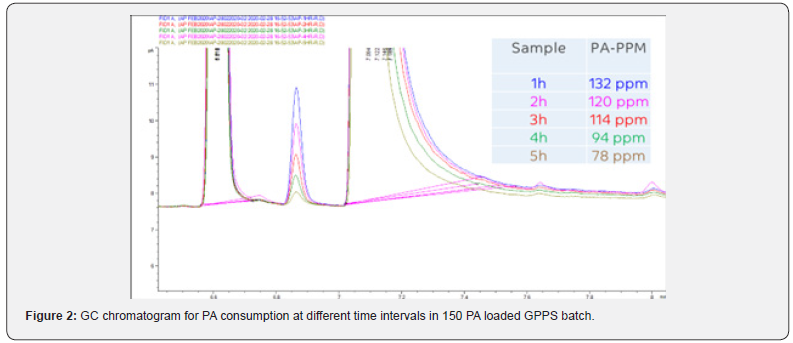
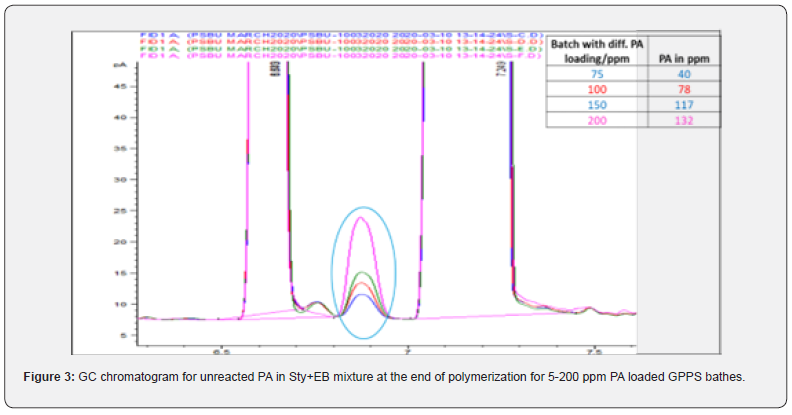
Moreover, PA did not consume completely even after 6 h of polymerization (Figure 2), it came to EB stream during devolatilization at above 50 ppm loading. This has confirmed in GC analysis of unreacted styrene+EB stream (Figure 3) collected at devolve stage, and the accumulation was increased at higher level of PA. Though there was some decrease in molecular weight at higher level of PA (>50 ppm), but there was no considerable deterioration in mechanical and thermal properties of GPPS (Table 1). The final extruded GPPS pellets did not contain any residual PA, which was confirmed by GC analysis. Lab values confirmed that there was no influence of PA on colour values.
HIPS
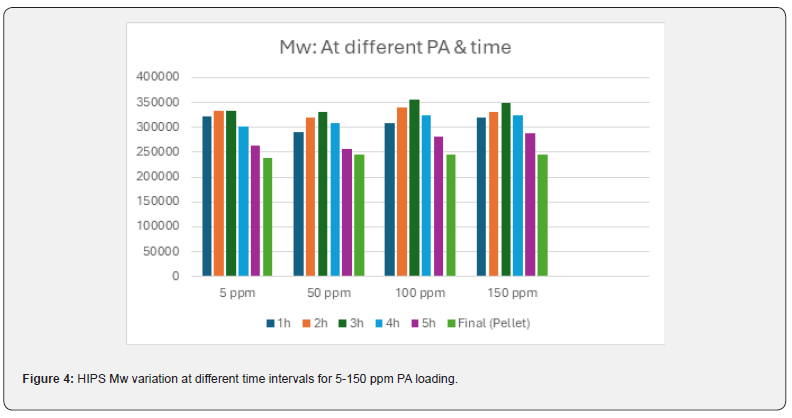
HIPS was prepared by dissolving 6.3% medium cis (40-42 %) PBR in styrene. Luperox 26M50 initiator was used for all the batches; only PA loading was changed from batch to batch, keeping reaction condition same. Total 5 batches were formulated from 5-150 ppm of PA. Interestingly, the final molecular weight was almost same for all the batches, irrespective of PA loading (Figure 4 & Table 2). The maximum molecular weight was achieved in 1-2 h at 5 ppm PA, whereas during 50-150 ppm PA loaded polymerization, maximum molecular weight achieved at 2-3 h. This delay in kinetics could be due to retardation of polymerization by comparatively stable radical produced with PA[1-6], same like in the case of GPPS. The amount of styrene collected at devolve increased with increase in PA concentration (Table 2), the fall in conversion could be up to 15-16 % at 100 ppm PA loading. There was no considerable changes in thermal properties (Tg, VST, MVR) as a function of PA loading as observed in (Table 2). The essential mechanical property in HIPS, the izod impact strength, mainly depends on few factors like quantity of rubber added, grafting of PS on rubber chain, gel content and rubber particles shape and distribution. In presence of PA at 50 ppm and above, the izod impact drastically reduced (Table 2) and same trend observed in gel content. Both impact and gel content dropped to 50 % less compared to 5 ppm PA batch and even further reduced at higher level of PA. PA chemically is known for a weak chain transfer agent and can convert active growing PS chain to dormant species in vinyl polymerization. Probably due to this, the grafting of PS chain on rubber abridged and not allowed to form enough cross-linking resulted in low gel content. This was further supported by TEM analysis of molded HIPS sample (Table 5). At lower level of PA (5 ppm), nice and stable salami structure was formed due to better grafting and gel. At 50 ppm and above a distorted shape and size of rubber particles led to poor mechanical property. The final color analysis showed not much batch-to-batch variations in Lab values. Although PA is light yellow liquid, chemically once reacted with PS chain, it will be structurally same as PS; hence it cannot impart any colour to HIPS or GPPS. From GC analysis, the absence of PA in final pellets were confirmed.
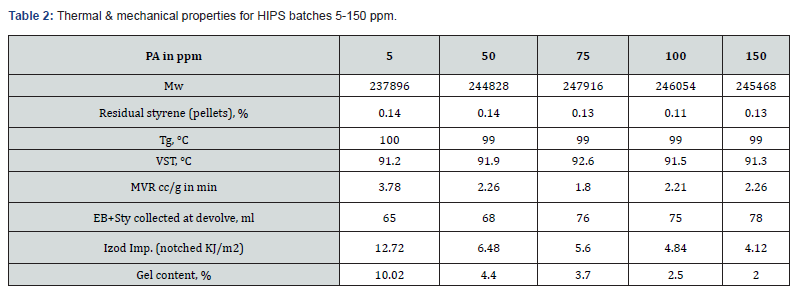
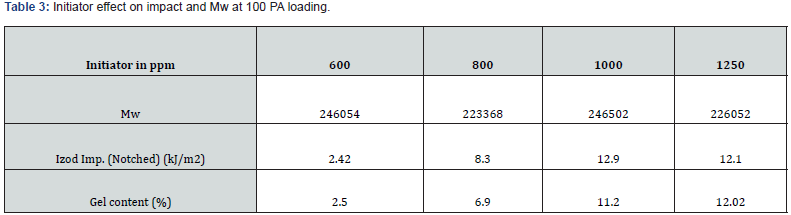
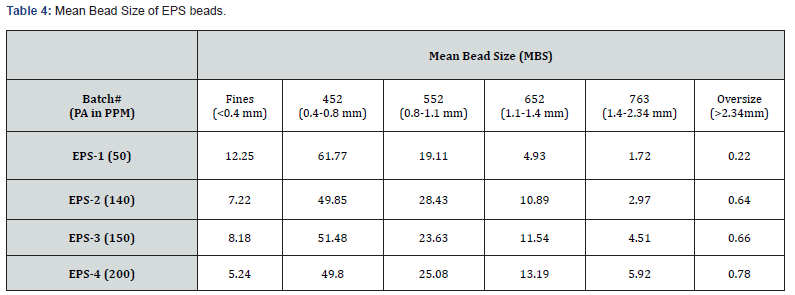
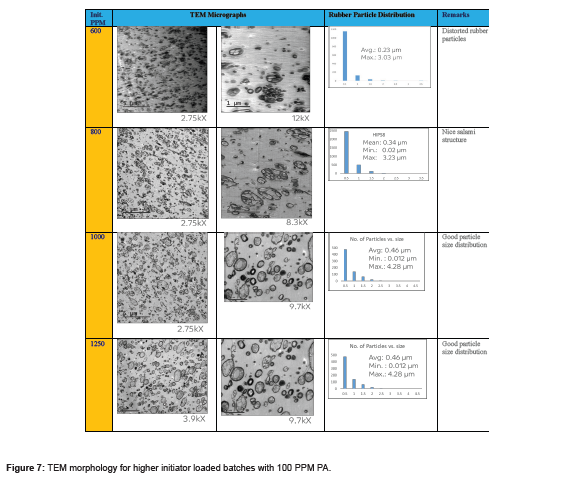
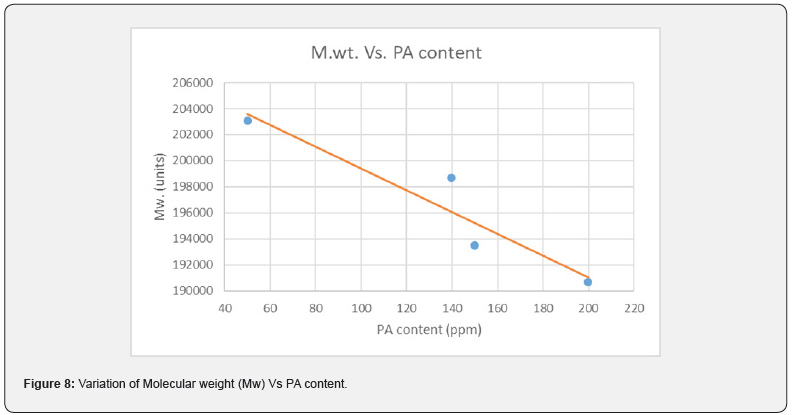
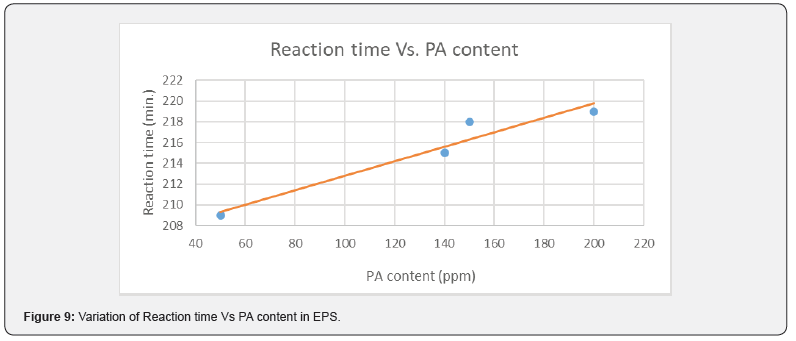
PA can be consumed or absorbed in polymerization process up to 50 ppm PA loading (Figure 5), this was confirmed by GC analysis of EB stream at devolve. That means, in 50 ppm PA batches, PA could not be find out in EB+styrene collected at the end of the reaction. Above 50 ppm a lot of unreacted PA came along with EB, and it can keep on accumulating in recycle stream in continuous process. To overcome this issue of PA particularly with HIPS process (assuming styrene supply will contain PA above 100 ppm), we thought of some practically feasible solutions. One is increasing the initiator concentration to compensate the retardation caused by PA during polymerization or grafting. Three experiments were conducted at higher initiator loading keeping all other ingredients same. The results are tabulated in Table 3. Excellent improvement in izod impact strength observed at 800- 1000 ppm initiator loading and same trend can be seen in gel content too. That means 300-400 % improvement in impact was seen by 200-400 ppm excess initiator addition. TEM analysis also confirmed very good salami structure and distribution (Figure 7). EPS was synthesized by a two-stage suspension polymerization of styrene using a combination of peroxide initiators in 30 L jacketed reactor. The first stage of the polymerization was carried out at 90 °C, and the second stage at 130 °C. The initiators were Benzoyl peroxide (BPO) and tert-butyl peroxybenzoate (TBP), for the first and second stage respectively. Total 4 experiments were conducted with PA content of 50, 140, 150 & 200 ppm. The molecular weight of EPS reduced as PA content increased (Figure 8). The reaction time and residual styrene in final EPS beads were seen to increase as PA content increased (Figure 9 & 10) respectively. On process side, though the suspension was stable throughout the polymerization at higher level of PA, but the Mean Bead Size (MBS) profile of final EPS is changed. From (Table 4) it is clear that as PA content increased in styrene the oversized beads increased considerably. More oversize beads may attributed to the slightly acidic nature of PA, which can shift the pH of the suspension towards acidic, consequently more oversize beads were obtained. Due to decrease in the molecular weight and change in the bead distribution, the overall final density of the EPS beads decreased around ~15-18 % at 150-200 PPM PA. Physical properties like compressive strength, flexural modulus and flexural strength of expanded EPS block are directly proportional to the density of pentane impregnated EPS beads. So, we can expect the deterioration of these properties at PA level >50 PPM (Table S1-S4).
Conclusion
In GPPS process, no external initiator was added and it was a thermal initiation process. There was a small drop in molecular weight (average 2-4 %) for every 50 ppm increase of PA, also there was a drop in conversion 5-7 % for every 50 ppm PA. Thermal, mechanical and color properties not changed considerably. HIPS polymerization environment is completely different from GPPS, where grafting and desired rubber particle formation is a key. In standard HIPS process, 600 ppm radical initiator is added which may not be enough to get desired impact property (~10 KJ/m2) assuming styrene supply will be having > 100 ppm PA. It is understood from our experiments that, PA above 50 ppm can deteriorate the mechanical properties (Table 2) if same initiator level or reaction conditions continued. Addition of 200-250 ppm of excess initiator can improve the impact performance without sacrificing other properties. As a second solution, temperature of pre-polymerization step can be increased 5-10 °C, so that more thermal initiator can be generated in styrene, but it may also change the reaction kinetics and reaction parameters. Up to 50 ppm, PA in styrene can absorb in polymerization and is not coming to recycle stream. For EPS production, it was necessary to maintain the PA level at 50 ppm, because above this level, oversized beads and residual styrene increased considerably.
References
- S (2022) National Library of Medicine, National Center for Biotechnology Infromation, PubChem, Compound Summary: Styrene.
- McKeen LW (2010) Fatigue and Tribological Properties of Plastics and Elastomers (2nd) Burlington, MA, USA, Elsevier.
- F Cavani, F Trifirò (1995) Applied Catalysis A: General 133 219-239.
- Distilation of Bulk Chemicals, Hendrik A. Kooijman, Ross Taylor, in Distillation: Operation and Applications, ScienceDirect, 2014.
- William L, Luyben (2011) Design and Control of the Styrene Process. Industrial & Engineering Chemistry Research
50 (3): 1231-1246.
- Suraj Vasudevan, GP Rangaiah, NVSN Murthy Konda, Wee Hwa Tay (2009) Application and Evaluation of Three Methodologies for Plantwide Control of the Styrene Monomer Plant. Industrial & Engineering Chemistry Research 48(24): 10941-10961.
- James R, Butler, Kevin P Kelly Fina Technology, Inc. US5156816.
- BA Wilhite, MJ McCready, A Varma (2002) Ind Eng Chem Res 41: 3345-3350.
- Morton Litt (1993) Polymer International 30: 213-216.
- Jűrgen Schellenberg (2005) Effect of Impurities on the Syndiospecific Coordination Polymerization of Styrene Macromolecular Materials and Engineering 290: 833-842.






























