Synthesis of A [5] Helicene-Based Chiral Polymer
Janet Olseon M and Thieo Hogen Esch E*
Department of Chemistry, University of Southern California, Loker Hydrocarbon Research Institute, USA
Submission: March 18, 2019; Published: March 18, 2019;
*Corresponding author: Thieo Hogen Esch E, Department of Chemistry, University of Southern California, Loker Hydrocarbon Research Institute, Los Angeles, CA90089-1661, USA
How to cite this article: Janet O M, Thieo H E. Synthesis of A [5] Helicene-Based Chiral Polymer. Academ J Polym Sci. 2019; 2(5): 555598.DOI: 10.19080/AJOP.2019.02.555598
Abstract
A copolymer precursor, an alkoxy-functionalized 3,12-diethyenyl [5] helicene, was prepared in seven consecutive steps. A Pd (0)-catalyzed reaction between this helicene derivative and p-diiodo benzene gave polymer 11, which is soluble in many common organic solvents. Peaks in the UV spectrum of 11 are shifted to the red when compared with the spectrum of monomeric analogue 12, presumably because of extended conjugation between the helicene comonomers and the aryl connector units.
Keywords: 3,12-diethyenyl [5] helicene; P-Diiodo Benzene; Organic Light-Emitting Diodes; Benzylidene; Helicene Polymer Precursors; Methyl Ether; Polymeric Helicenes; Naphthyl Groups
Introduction
Helicenes are polycyclic structures composed of n ortho-fused aromatic rings, where n refers to the number of rings. When n reaches five or more, the molecule experiences steric repulsion between the terminal rings, causing the molecule to twist into a helix [1]. However, some phenanthrenes and benzo phenanthrene were optically resolved and conformationally stable since the early days of helicene chemistry (n = 3 & 4). The inherent chirality of these molecules gives rise to large optical rotations (several thousand degrees) [2] and high circular dichroism values. [3] As chiral conjugated compounds, helicenes and their derivatives have found applications, including semiconductors [4,5] organic light-emitting diodes (OLEDs), [6,7] and nonlinear optical (NLO) materials [8].
The above reports have all dealt with helicenes on the molecular level, i.e. small molecules. The synthesis of the corresponding polymeric helicenes is well known not to be feasible as the steric congestion has been shown to prevent the formation of helicenes longer than [14] helicene [9]. However, the synthesis of [16] helicenes containing alternating copolymers having a vinylene [10] or aryl [11] connector “connector” is possible [11]. Such structures could feature both extended partial conjugation and the helicene chirality. Pu and co-workers synthesized many polymers in which 1,1’-binaphthyls alternated with aryl groups to produce structures with main-chain chirality [12]. However, extended conjugation across the binaphthyl 1,1’-moiety in a polymer is essentially absent given the large dihedral angle between the two naphthyl groups [13].
Unlike 1,1’-binaphthalene, partial conjugation in helicenes can extend across the entire molecule [11] Moreover, copoly merization with a benzylidine or similar arylidene connector group should allow increased delocalization. However, helicenes with polymerization potential represent a more synthetically challenging target [11,14,15]. Hence, the synthesis of new and more effective helicene polymer precursors is of interest.
The route toward a new helicene monomer was inspired by a 2007 report by Kamikawa and co-workers. The focus of this work was the one-step conversion of Z, Z-stilbenes into helicenes, via a palladium-mediated C-H arylation reaction (Figure 1) [16].
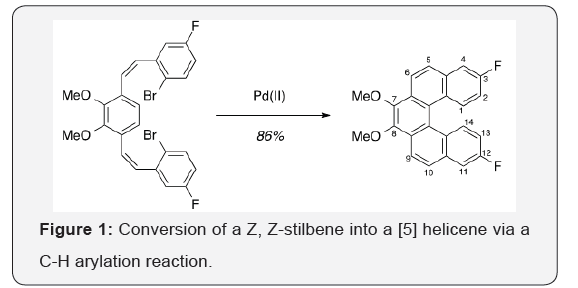
Upon replacement of the fluorine in this reported helicene with an acetylene group, reaction with an aryl halide to generate a fully conjugated polymer under Sonogashira reaction conditions would be possible [17,18]. While the direct conversion of aryl fluorides into acetylenes is difficult, replacing fluorine with chlorine should make this reaction possible [19].
The methoxy groups on this helicene offer another opportunity for modification. Although weaker than for angular or
planar polymers, π-π interactions in helicenes could render these polymers insoluble in the common organic solvents used for processing and characterization. This can be addressed by including solubilizing groups, such as long alkyl chains [20]. Cleavage of the methyl ether, followed by alkylation with a longer chain will help to ensure that the resulting polymer will stay dissolved during its polymerization and subsequent processing, if needed. Additionally, the methoxy functional group serves as an electron-donor (D). The general D-π-A (where π is a conjugated linker, and A is an electron-acceptor) structure has been determined to be effective in NLO polymers, [21] and is also desirable for modification of electronic properties through tuning of the band gap [22]. An aryl group functionalized with A groups and then polymerized with a D-containing helicene could give an extended structure of these D-π-A units.
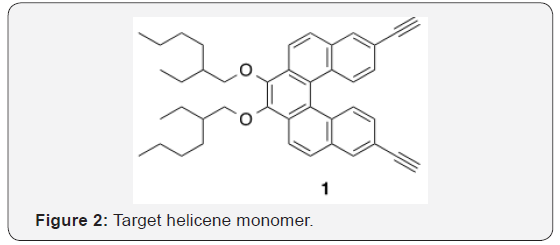
Here we report our route toward helicene derivative 1. However, the racemization barrier for [5] helicene (24.1 kcal/mol at 27 °C) [23] is low enough that it racemizes at room temperature within a few hours [24]. Because of this, the synthesis of a [5] helicene- based chiral (but not necessarily configurationally stable) polymer is not the main objective, but rather, a demonstration of a viable synthetic route Figure 2.
Experimental section
All reactions, unless otherwise noted, were conducted using commercially available solvents and reagents as received, without additional purification, in ordinary glassware under an inert argon atmosphere. Veratrole and TEA were distilled after being stirred over CaH2 for several hours. Dry solvents, such as: DCM, DMF, and THF were obtained from a DriSolv® bottle. 1H & 13C NMR spectra were recorded on Mercury 400 or Varian 400-MR (400MHz) NMR spectrometers, using residual 1H or 13C signals of deuterated solvents as internal reference standards. Reactions were monitored by TLC carried out on 0.200mm analytical layer Baker-flex® plates using UV light (254nm) as the visualizing agent. Silica gel (60Å, 40-63μm; Alfa Aesar) was used as a sorbent for flash column chromatography.
2,3-Dimethoxy-1,4-dicarbaldehyde (5). TMEDA (37.5ml, .250 mol) was added to a solution of veratrole (6.37ml, .0500mol) in diethyl ether (DEE) (250ml) [3]. The reaction mixture was cooled to -78 °C, and n-butyllithium 2.5M in hexanes (100ml, .250mol) was added over 10min. The solution could warm to rt, heated to reflux, and allowed to stir for 16h. The solution was then cooled to -78 °C for the addition of DMF (19.3mL, 0.250mol), followed by warming to rt and stirring for 4h. Finally, the 200ml of water was added, followed by the addition of 3M HCl to neutralize the solution. The organic layer was separated, and the aqueous layer was extracted using three 500ml portions of DEE. The combined organic layers were washed with saturated aqueous NaHCO3, water, and brine, followed by drying over Na2SO4 and concentration under reduced pressure. Purification by flash column chromatography using 25% EtOAc/hexanes as eluent, followed by recrystallization from 10% toluene/hexanes afforded a pale yellow solid (3.31g, 34%). TLC: 25% EtOAc/hexanes, Rf ≈ 0.5. Mp 98-100 °C. 1H NMR (400MHz, CDCl3) δ 10.44 (s, 2H), 7.62 (s, 2H), 4.05 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 189.37, 156.79, 134.36, 122.98, 62.61.
(2-Bromo-5-chlorobenzyl) triphenylphosphonium bromide [3]. 1-Bromo-2-(bromomethyl)-4-chloro-benzene (2.50g, 8.79mmol) and triphenylphosphine (2.31g, 8.79mmol) were brought up in DMF (9ml) and allowed to stir at reflux for 3h.1,2 The mixture was allowed to cool to rt and filtered to collect the product which was washed with cold toluene, followed by cold hexanes. The product was obtained as a colorless solid (4.73g, 98%). Mp > 250 °C. 1H NMR (400MHz, CDCl3) δ 7.85-7.50 (m, 15H), 7.47 (q, J = 2.6 Hz, 1H), 7.30 (d, J = 8.5Hz, 1H), 7.11 (dt, J = 8.8, 2.6Hz, 1H), 5.83 (d, J = 15.0Hz, 2H). 13C NMR (101MHz, CDCl3) δ 135.39 (d, JPC = 3.1Hz), 134.53 (d, JPC = 10.0Hz), 133.95 (d, JPC = 3.4Hz), 133.04 (d, JPC = 4.9Hz), 130.44 (d, JPC = 12.8Hz), 129.95 (d, JPC = 8.8Hz), 125.18 (d, JPC = 6.7Hz), 117.50 (d, JPC = 86.0Hz), 31.17 (d, JPC = 48.6Hz) (two peaks are not resolved).
1,4-Bis[(1Z)-2-(2-bromo-5-chlorophenyl) ethenyl]-2,3-dimethoxybenzene [6]. A solution of 5 (1.06g, 5.45 mmol) and 3 (6.56g, 12.0mmol) in THF (44ml) was cooled to 0°C [4]. Potassium tert-butoxide (1.46g, 12.4 mmol) dissolved in water (4.4ml) was added, dropwise, followed by warming to rt and stirring for 30min. The resulting mixture was partitioned between EtOAc and water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Purification by flash column chromatography using 10% EtOAc/hexanes as eluent, followed by recrystallization from 10% toluene/hexanes afforded a colorless solid (2.10g, 68%). TLC: 14% EtOAc/Hexanes, Rf ≈ 0.7.Mp 114- 116 °C. 1H NMR (400MHz, CDCl3) δ 7.47 (d, J = 8.5Hz, 2H), 7.10 (d, J = 2.6 Hz, 2H), 7.02 (ddd, J = 8.6, 2.6, 0.6 Hz, 2H), 6.82 (d, J = 12.1 Hz, 2H), 6.58 (d, J = 12.0 Hz, 2H), 6.50 (s, 2H), 3.84 (s, 6H). 13C NMR (101MHz, CDCl3) δ 151.62, 139.51, 133.83, 133.04, 130.35, 130.33, 129.16, 128.78, 127.38, 124.37, 121.73, 61.07.
8,13-Dichloro-3,4-dimethoxydibenzo[c,g]phenanthrene [7]. Pd(OAc)2 (78.7mg, 0.351mmol), K2CO3 (0.972g, 7.03mmol), Ag2CO3 (0.485g, 1.76mmol), PCy3·HBF4 (0.277g, 0.703mmol), and 2.96 (2.00g, 3.51mmol) were brought up in DMA (66.8ml) and allowed to stir at 120 °C for 16h [4]. Upon completion, the solution was cooled to rt and filtered through a pad of Celite to remove insoluble salts. The filtrate was concentrated under reduced pressure and purified by flash column chromatography using 20% DCM/hexanes as eluent to afford a pale yellow solid (0.900 g, 63%). TLC: 75% DCM/hexanes, Rf ≈ 0.7. Mp 231-233 °C. 1H NMR (400MHz, CDCl3) δ 8.31 (d, J = 8.8Hz, 2H), 8.27 (d, J = 9.2Hz, 2H), 7.92 (d, J = 2.1Hz, 2H), 7.86 (d, J = 8.9Hz, 2H), 7.21 (dd, J = 9.0, 2.3Hz, 2H), 4.13 (s, 6H). 13C NMR (101MHz, CDCl3) δ 145.32, 133.21, 131.76, 130.39, 128.98, 128.72, 126.97, 126.82, 125.40, 124.15, 121.24, 61.32.
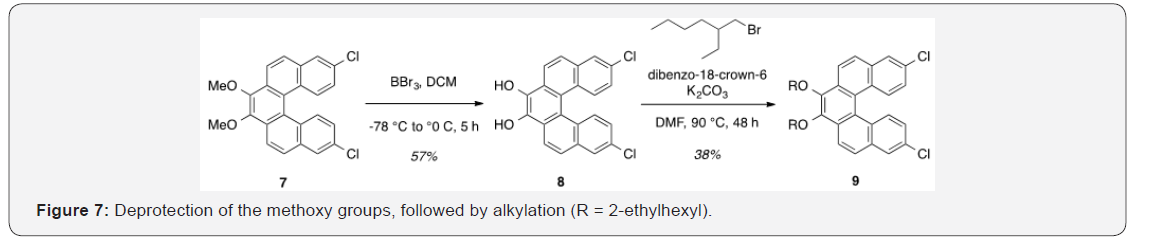
8,13-Dichlorodibenzo[c,g]phenanthrene-3,4-diol [8]. A solution of 2.107 (0.603g, 1.48mmol) in DCM (16ml) was cooled to -78 °C for dropwise addition of 1.0M BBR3 in DCM (3.7ml, 3.70mmol).5,6 After 30min, the reaction was allowed to warm to rt and stirred for 16h. The mixture was then cooled to 0 °C and quenched with water, followed by extraction of the aqueous layer with DCM. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Purification by flash column chromatography using 20% hexanes/DCM as eluent afforded a red solid (0.320g, 57%). TLC: 33% hexanes/DCM, Rf ≈ 0.5. Mp > 250 °C. 1H NMR (400MHz, CDCl3) δ 8.20 (d, J = 8.4Hz, 2H), 7.98-7.90 (m, 4H), 7.40 -7.28 (m, 4H). 13C NMR (101MHz, CDCl3) δ 182.73, 138.35, 137.16, 135.86, 130.22, 129.57, 128.80, 128.50, 127.67, 127.63, 124.91.
8,13-Dichloro-3,4-bis[(2-ethylhexyl) oxy] dibenzo[c,g] phenanthrene [9]. To a solution of 2.118 (0.228g, 0.601 mmol), K2CO3 (0.498g, 3.61mmol), and dibenzo-18-crown-6 (0.0220g, 0.0601mmol) in DMF (4ml), 2-ethylhexyl bromide (0.640ml, 4.81mmol) was added.7 The reaction was heated to 90 °C and allowed to stir for 48 h. The resulting mixture was cooled to rt and partitioned between DCM and water, followed by extraction of the aqueous layer with DCM. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Purification by flash column chromatography using 4% DCM/hexanes afforded a yellow oil (0.139g, 38%). TLC: 9% DCM/hexanes, Rf ≈ 0.5. 1H NMR (400MHz, CDCl3) δ 8.34 (d, J = 8.9Hz, 2H), 8.28 (d, J = 9.0Hz, 2H), 7.92 (d, J = 2.2Hz, 2H), 7.86 (d, J = 8.8Hz, 2H), 7.21 (dd, J = 9.0, 2.2Hz, 2H), 4.31 (m, 2H), 3.98 (m, 2H), 1.92m, 2H), 1.83-1.48 (m, 8H), 1.48-1.30 (m, 8H), 1.03 (td, J = 7.5, 2.1 Hz, 6H), 0.95 (dt, J = 6.6, 3.3Hz, 6H). 13C NMR (101MHz, CDCl3) δ 144.86, 133.12, 131.60, 130.43, 129.13, 129.03, 126.79, 126.79, 125.32, 124.01, 121.42, 76.97, 40.85, 30.66, 29.31, 24.07, 23.30, 14.28, 11.38.
3,4-Bis[(2-ethylhexyl) oxy]-8,13-bis(triisopropylsilylethynyl) dibenzo[c,g]-phenanthrene [10]. To a flask containing [PdCl2(CH3CN)2] (13.7mg, 0.0524mmol), XPhos (0.153g, 0.314mmol), and Cs2CO3 (2.22g, 6.81 mmol) was added a solution of 2.129 (0.791g, 1.31mmol) in anhydrous 1,4-dioxane (5.2ml) [8]. The suspension could stir at rt for 25min prior to the addition of (triisopropylsilyl)acetylene (1.22ml, 5.24mmol). The mixture was heated to 90°C and allowed to stir for 16h. After cooling to rt, the reaction was diluted with water and extracted with DEE. The combined organic layers were washed with brine, dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using 20% hexanes/ DCM as eluent afforded a yellow oil (0.940g, 80%). TLC: 10% DCM/hexanes, Rf ≈ 0.6. 1H NMR (400MHz, CDCl3) δ 8.33 (dd, J = 16.5, 8.9Hz, 4H), 8.14 (d, J = 1.7Hz, 2H), 7.93 (d, J = 8.8Hz, 2H), 7.38 (dd, J = 8.8, 1.7Hz, 2H), 4.36 (m, 2H), 4.00 (m, 2H), 1.96 (m, 2H), 1.83-1.55 (m, 8H), 1.49-1.38 (m, 8H), 1.24 (s, 42H), 1.07 (td, J = 7.4, 2.1Hz, 6H), 0.98 (td, J = 7.0, 2.0Hz, 6H). 13C NMR (101MHz, CDCl3) δ 145.04, 131.88, 131.74, 130.36, 129.45, 128.96, 127.81, 127.35, 124.29, 120.91, 120.81, 107.51, 91.35, 76.95, 40.89, 30.68, 29.34, 24.11, 23.34, 18.93, 14.30, 11.60, 11.41.
3,4-Bis((2-ethylhexyl) oxy)-8,13-diethynyldibenzo[c,g] phenanthrene [1]. A solution of 2.1310 (0.999g, 1.12mmol) in THF (22.3ml) was cooled to 0°C [9] TBAF (1.0M in THF) was added dropwise and the solution could warm to rt. After stirring for 16h, saturated aqueous NaHCO3 was added to the mixture, which was then extracted with DEE. The combined organic layers were washed with water, dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using 16% hexanes/DCM as eluent afforded a yellow oil (0.559g, 96%). TLC: 25% DCM/hexanes, Rf ≈ 0.7. 1H NMR (400MHz, CDCl3) δ 8.36 (d, J = 8.8Hz, 2H), 8.31 (d, J = 8.8Hz, 2H), 8.14 (d, J = 1.8Hz, 2H), 7.92 (d, J = 8.7Hz, 2H), 7.36 (dd, J = 8.8, 1.5Hz, 2H), 4.39-4.28 (m, 2H), 4.06-3.95 (m, 2H), 3.20 (s, 2H), 1.99-1.92 (m, 2H), 1.85-1.52 (m, 8H), 1.48-1.37 (m, 8H), 1.06 (td, J = 7.5, 2.2Hz, 6H), 1.01-0.93 (m, 6H). 13C NMR (101MHz, CDCl3) δ 145.09, 132.18, 131.65, 130.55, 129.58, 129.07, 127.59, 127.33, 124.17, 120.94, 119.46, 83.98, 77.89, 76.94, 40.84, 30.65, 29.31, 24.07, 23.30, 14.27, 11.38. UV-Vis absorption maxima: 258 (ε = 83,300 L mol-1 cm-1), 325 (ε = 48,100 L mol-1 cm-1), and 368 (ε = 8,500 L mol-1 cm-1) nm.
3,4-Bis((2-ethylhexyl) oxy)-8,13-bis(phenylethynyl)dibenzo[ c,g]phenanthrene [13]. A flask charged with 2.11 (72.5mg, 0.129mmol), tetrakis(triphenylphosphine)palladium (0) (7.4mg, 6.43μmol), and copper(I) iodide (1.2mg, 6.43μmol) was brought up in THF (1.3ml) [10]. Iodobenzene (43.3μl, 0.387mmol) and TEA (0.77ml) were then added and the reaction could stir at 40°C for 16h. The reaction mixture was then cooled to rt and filtered with DCM through a short silica gel column, followed by concentration of the solvent under reduced pressure. Purification by flash column chromatography with 8% EtOAc/hexanes as eluent afforded a yellow oil (90.2 mg, 95%) TLC: 17% EtOAc/ hexanes, Rf ≈ 0.8. 1H NMR (400MHz, CDCl3) δ 8.33 (d, J = 8.8Hz, 4H), 8.15 (d, J = 1.8Hz, 2H), 7.92 (d, J = 8.8Hz, 2H), 7.64-7.55 (m, 4H), 7.43-7.33 (m, 8H), 4.36-4.25 (m, 2H), 4.01-3.95 (m, 2H), 1.96-1.89 (m, 2H), 1.82-1.45 (m, 8H), 1.45-1.32 (m, 8H), 1.02 (td, J = 7.5, 2.1Hz, 6H), 0.94 (ddt, J = 7.2, 4.9, 1.7Hz, 6H). 13C NMR (101MHz, CDCl3) δ 145.06, 131.83, 131.81, 131.34, 130.29, 129.45, 129.12, 128.53, 128.44, 127.39, 124.27, 123.46, 120.87, 120.64, 90.26, 89.83, 76.96, 40.85, 30.66, 29.31, 24.08, 23.31, 14.28, 11.39. UV-Vis absorption maxima: 283 (ε = 50,900 L mol- 1 cm-1), 332 (ε = 49,900 L mol-1 cm-1), and 372 (ε = 17,700 L mol-1 cm-1) nm.
Polymerization. A mixture of 2.11 (58.9mg, 0.101mmol), p-diiodobenzene (33.7mg, 0.101mmol), tetrakis(triphenylphosphine) palladium(0) (5.8mg, 5.05μmol), and copper(I) iodide (1.0mg, 5.05μmol) were dissolved in THF (10ml) and TEA (6ml) [11]. The solution was heated to 40°C and allowed to stir for 48h. Phenylacetylene (11μl, 0.101mmol) was then added and the solution continued to stir for 1h. The reaction was then cooled to rt and filtered through a short silica plug. The solvent was removed under reduced pressure and the resulting polymer was precipitated in MeOH. The precipitated polymer was dried under high vacuum for 48h at 60 °C, resulting in 50.0mg (75%) of an orange solid. Mn = 4,411 g/mol, Mw = 6,531 g/mol, PDI = 1.48. UV-Vis absorption maxima: 286 (ε = 57,100 L mol-1 cm- 1), 348 (ε = 85,100 L mol-1 cm-1), and 382 (ε = 43,300 L mol-1 cm-1) nm.
Characterization
SEC was performed on a Shimadzu HPLC system consisting of: a Shimadzu LC-20AT HPLC pump, a Rheodyne 7725i injector, Phenogel 5u 50 x 7.8mm Guard Column, Polymer Laboratories PLgel 5μm MIXED-C column x 2, and a Shimadzu RID 10-A detector, at ambient temperature, using THF (HPLC grade) as the elution solvent with a flow rate of 1 ml/min. Polystyrene standards were used for calibration.
The absorbance spectra were recorded on an Agilent UV-Visible Spectrophotometer using a 1cm trajectory and a blank cell (CHCl3) for each sample. Monomer 2.1 (4.4mg) was dissolved in 5ml of CHCl3 to make a stock solution (3.69x10-4 M), which was then diluted to achieve an appropriate concentration for measurement (1.42x10-5M). Model monomer 2.14 (1.6mg) was dissolved in 4 ml of CHCl3 to make a stock solution (5.45x10-4M), which was then diluted to achieve an appropriate concentration for measurement (4.95x10-5M M). Polymer 2.15 (0.4mg) was dissolved in 4ml of CHCl3 to make a stock solution (2.01x10- 5M), which was then diluted to achieve an appropriate concentration for measurement (1.82x10-6M).
Results and Discussion
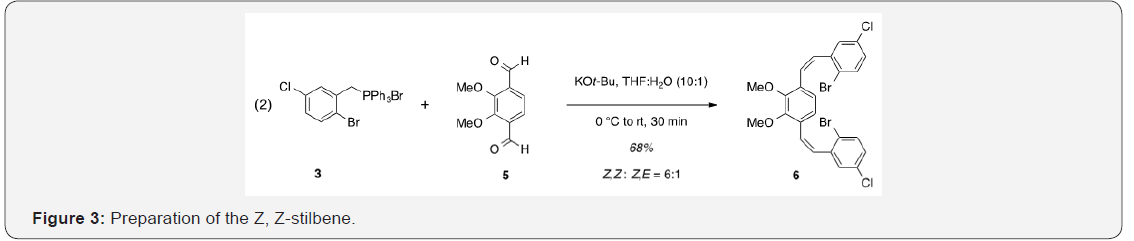
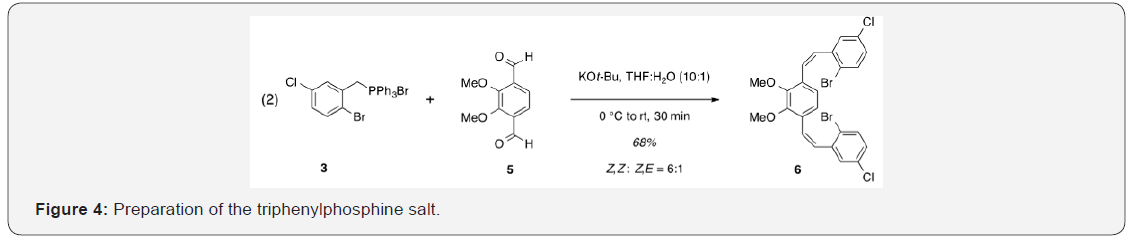
The synthesis of target structure 1 begins with the assembly of a Z, Z-stilbene (Figure 3), which is, in turn, the precursor for the C-H arylation reaction (Figure 4). The required triphenylphosphine salt 3 was synthesized (Figure 5) by reaction of commercially available 2 with triphenylphosphine in near quantitative yield [25,26].
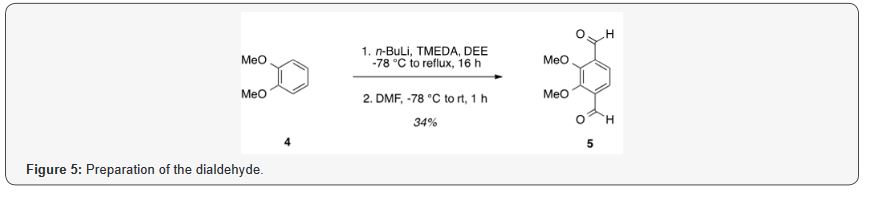
Dialdehyde 5 was prepared according to previous reports, via double directed ortho-lithiation of veratrole [4] [27] The relatively low yield of this reaction could be was likely due to the reduced solubility of the aryllithium intermediate or, possibly, secondary reactions, thus compromising the second lithiation [28]. Varying the conditions have not improved this low yield Figure 6.
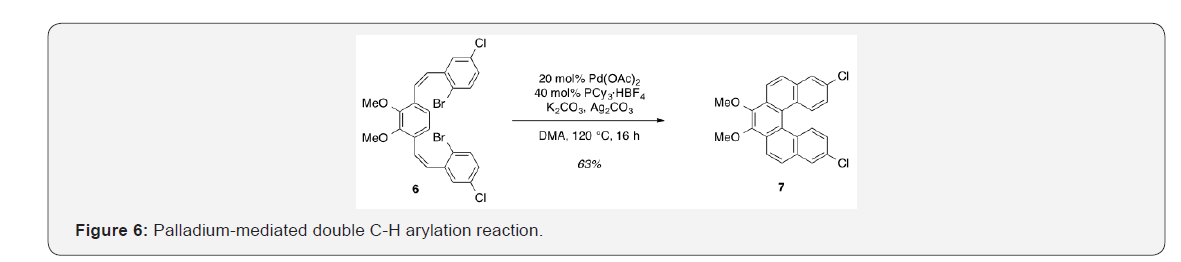
With both aldehyde 5 and triphenylphosphine salt 3 in hand, the two were combined under basic conditions to generate stilbene 6 (Figure 3) in a ratio of 6:1 Z, Z: Z,E.15 The subsequent conversion of 6 into 7, via a Pd (0)-catalyzed double C-H arylation reaction was accomplished in 63% yield (Figure 4). Because helicene 7 has limited solubility in many solvents, this intermediate was transformed into 9, having two 2-ethylhexyl groups [29]. This was carried out by cleavage of the methoxy ether functionality using boron tribromide [30,31] The resulting diol [8] was subsequently alkylated with 2-ethylhexyl bromide. This reaction proceeded slowly, but with the inclusion of dibenzo-18- crown-6 as a catalyst, a moderate yield (38%) of the alkylated product [9] was obtained as a viscous oil [32] Compound 9 was significantly more soluble in common organic solvents in comparison with its precursordecessor, 7. As pointed out above, the ability to vary the type of alkoxy group allows for a degree of leverage with respect to physical properties [8,22] Figure 7.
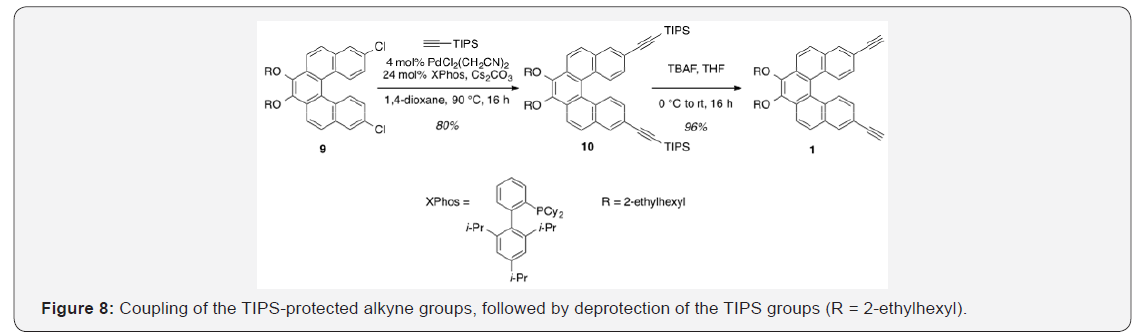
The last step in completing the helicene monomer 1 was the substitution of the two chlorinegs of 9 with alkyne groups that should allow copolymerization using Sonogashira conditions. Using the method reported by Buchwald,18 a Pd (II)-catalyzed substitution gave 10 having two TIPS-protected acetylene groups (Figure 8). In this case, doubling the ratio of ligand to metal (6:1 rather than 3:1) was necessary for optimal yields. A tetrabutylammonium fluoride-mediated deprotection of the TIPS groups gave monomer 1 in near quantitative yield [33].
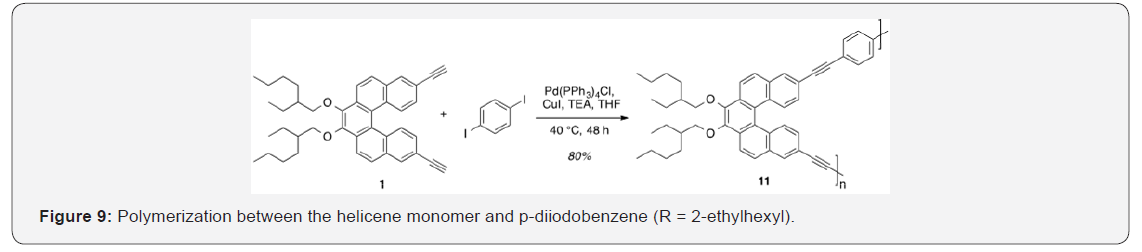
Monomer 1 was subjected to a Pd (0)-catalyzed Sonogashira reaction with p-diiodobenzene in the presence of catalytic amounts of Pd (PPh3)4Cl and CuI in THF and TEA to afford polymer 11 in 80% yield [17]. This polymer was soluble in common organic solvents, including THF, DCM, and CHCl3. The number-average molecular weight (Mn) was determined to be 4,411g/mol (PDI = 1.48) by SEC, using polystyrene standards in THF [34,35] Figure 9. In addition, a model analogue 12 was synthesized by end-capping of 1 using similar reaction conditions, 16 allowing comparisons with polymer 11 (Figure 10).
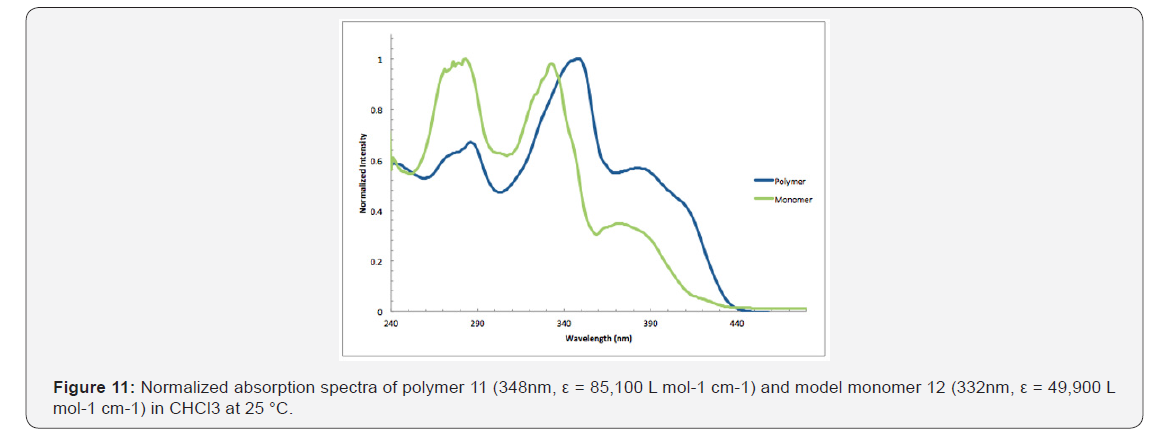
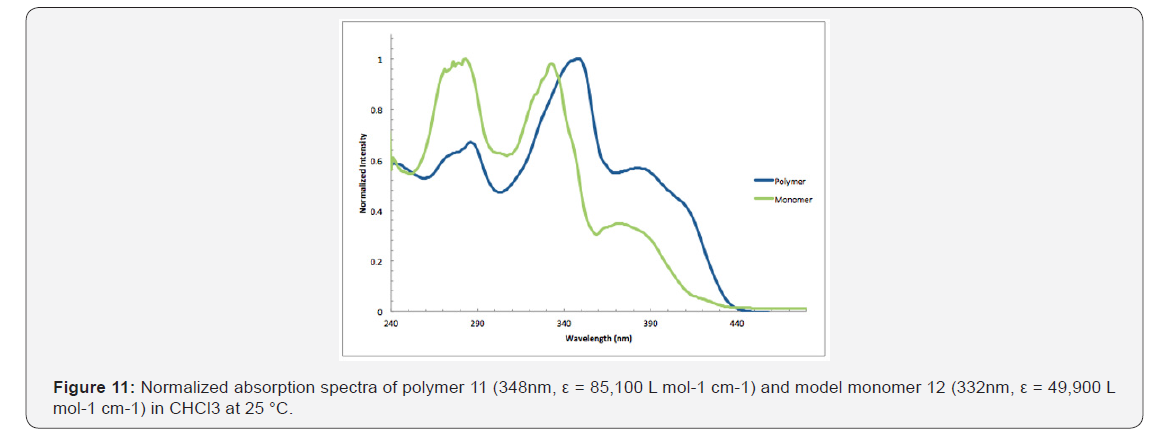
As shown in Figure 11, the absorption maxima at 286, 348, &382nm of polymer 11 are red shifted compared with model monomer 12 having maxima at 283, 332, and 372nm, with red shifts of 3, 16, & 12nm, respectively. These spectral differences are consistent with the extended partial π conjugation [36]. This is significant, because extended conjugation is a desired characteristic in the design of many NLO and other organic materials [37,38].
Conclusion
A 3,12-diethyenyl [5] helicene with electron-donating ether groups was prepared and copolymerized with an aryl connector to produce a polymer with extended conjugation. This is the first example of a polymerization of a helicene with modifiable electron- donating functionality. This variability opens up helicenes as potential tunable components in organic electronic materials. Efforts in our laboratory toward more conformationally stable helicene monomers are currently underway.
Acknowledgment
JO acknowledges support for this work by several Moulton fellowships by the Loker Hydrocarbon Research institute.
References
- Balaban AT (2003) Theoretical Examination of New Forms of Carbon Formed by Intra-or Intermolecular Dehydrogenation of Polycyclic Aromatic Hydrocarbons, Particularly Helicenes. Polycycl Aromat Compd 23: 277.
- Newman MS, Lednicer D (1956) The Synthesis and Resolution of Hexahelicene. J Am Chem Soc 78(18): 4765-4770.
- Gingras M (2013) One hundred years of helicene chemistry. Part 1: non-stereoselective syntheses of carbohelicenes. Chem Soc Rev 42(3): 968-1006.
- Yang Y, Da Costa RC, Fuchter MJ, Campbell A (2013) Circularly polarized light detection by a chiral organic semiconductor transistor. J Nat Photonics 7: 634-638.
- Hatakeyama T, Hashimoto S, Oba T, Nakamura M (2012) Spotlights on Recent JACS Publications. J Am Chem Soc 134(48): 19600-19950.
- Sahasithiwat S, Mophuang T, Menbangpung L, Kamtonwong S, Sooksimuang T (2010) 3,12-Dimethoxy-7,8-dicyano- [5] helicene as a novel emissive material for organic light-emitting diode. Synthetic Metals 160(11-12): 1148-1152.
- Shi L, Liu Z, Dong G, Duan L, Qiu Y, et al. (2012) Synthesis, structure, properties, and application of a carbazole-based diaza[7]helicene in a deep-blue-emitting OLED. Chemistry 18(26): 8092-8099.
- Verbiest T, Elshocht SV, Kauranen M, Hellemans L, Snauwaert J, et al. (1998) Strong enhancement of nonlinear optical properties through supramolecular chirality. Science 282(5390): 913-915.
- Martin RH, Baes M (1975) Helicenes: Photosyntheses of [11], [12] and [14] helicene. Tetrahedron 31(17): 2135-2137.
- Mori K, Murase T, Fujita M (2015) One‐Step Synthesis of [16] Helicene. Angew Chem Int Ed 54(23): 6847-6851.
- Dai Y, Katz TJ, Nichols DA (1996) Synthesis of a Helical Conjugated Ladder Polymer. Angewandte Chemie International Edition 35(18): 2109-2111.
- Pu L (2000) Novel chiral conjugated macromolecules for potential electrical and optical applications. Macromol Rapid Commun 21(12): 795-809.
- Pu L (1998) 1,1‘-Binaphthyl Dimers, Oligomers, and Polymers: Molecular Recognition, Asymmetric Catalysis, and New Materials. Chem Rev 98(7): 2405-2494.
- Takahira Y, Sugiura H, Yamaguchi M (2006) Synthesis and ring size effect of macrocyclic ethynylhelicene oligomers. J Org Chem 71(2): 763-767.
- Fox JM, Lin D, Itagaki Y, Fujita T (1998) Synthesis of Conjugated Helical Acetylene-Bridged Polymers and Cyclophanes. J Org Chem 63(6): 2031-2038.
- Kamikawa K, Takemoto I, Takemoto S, Matsuzaka H (2007) Synthesis of Helicenes Utilizing Palladium-Catalyzed Double C−H Arylation Reaction. J Org Chem 72(19): 7406-7408.
- Yuan WZ, Hu R, Lam JWY, Xie, N, Jim CKW, et al. (2012) Conjugated hyperbranched poly(aryleneethynylene)s: synthesis, photophysical properties, superquenching by explosive, photopatternability, and tunable high refractive indices. Chemistry 18(10): 2847-2856.
- Kondo K, Okuda M, Fujitani T (1993) Synthesis and nonlinear optics of soluble poly(phenyleneethynylene. Macromolecules, 26(26): 7382.
- Gelman D, Buchwald SL (2003) Efficient palladium-catalyzed coupling of aryl chlorides and tosylates with terminal alkynes: use of a copper cocatalyst inhibits the reaction. Angew Chem Int Ed Engl 42(48): 5993- 5996.
- Ma L, Hu QS, Musick KY, Vitharana D, Wu C, et al. (1996) Conjugated Polymers with Main Chain Chirality. 3. Synthesis of Optically Active Poly(aryleneethynylene). Macromolecules 29(15): 5083-5090.
- Dalton L (2002) Nonlinear Optical Polymeric Materials: From Chromophore Design to Commercial Applications. In Polymers for Photonics Applications I, Advances in Polymer Science, Springer Berlin Heidelberg 158: 1-86.
- Roncali (1997) Synthetic Principles for Bandgap Control in Linear π-Conjugated Systems. J Chem Rev 97(1): 173-206.
- Martin RH, Marchant MJ (1974) Thermal racemisation of hepta-, octa- , and nonahelicene : Kinetic results, reaction path and experimental proofs that the racemisation of hexa- and heptahelicene does not involve an intramolecular double diels-alder reaction. Tetrahedron 30(2): 347-349.
- Stammel C, Fröhlich R, Wolff C, Wenck H, De Meijere A, et al. (1999) Synthesis and X-ray analysis of new [5] helicenes - HMO calculations on the photocyclization of the stilbene precursors [1]. J European Journal of Organic Chemistry 7: 1709-1718.
- John Plater MJ (1997) Chem Soc Perkin Fullerene tectonics. Part 2.1 Synthesis and pyrolysis of halogenated benzo[c]phenanthrenes 19: 2903-2909.
- Caruso A, Siegler MA, Tovar JD (2010) Synthesis of Functionalizable Boron‐Containing π‐Electron Materials that Incorporate Formally Aromatic Fused Borepin Rings. Angew Chem Int Ed 49(25): 4213- 4217.
- Kuhnert N, Rossignolo GM, Lopez Periago A (2003) The synthesis of trianglimines: on the scope and limitations of the [3 + 3] cyclocondensation reaction between (1R,2R)-diaminocyclohexane and aromatic dicarboxaldehydes. Org Biomol Chem 1: 1157-1170.
- Crowther GP, Sundberg RJ, Sarpeshkar AM (1984) Dilithiation of aromatic ethers J Org Chem 49: 4657-4663.
- Padmanaban G, Ramakrishnan S (2000) Conjugation Length Control in Soluble Poly[2-methoxy-5-((2‘-ethylhexyl) oxy)-1,4- phenylenevinylene] (MEHPPV): Synthesis, Optical Properties, and Energy Transfer. J Am Chem Soc 122(10): 2244-2251.
- Nakajima M, Miyoshi I, Kanayama K, Hashimoto SI (1999) Enantioselective Synthesis of Binaphthol Derivatives by Oxidative Coupling of Naphthol Derivatives Catalyzed by Chiral Diamine·Copper Complexes. J Org Chem 64: 2264-2271.
- Bandind M, Casolari S, Cozzi PG, Proni G, Schmohel E, et al. (2000) Synthesis and Characterization of New Enantiopure 7,7′‐Disubstituted 2,2′‐Dihydroxy‐1,1′‐binaphthyls: Useful Ligands for the Asymmetric Allylation Reaction of Aldehydes. A European Journal of Organic Chemistry 491-497.
- Howard MJ, Heirtzler FR, Dias SIG (2008) Synthesis and Stereochemistry of Long-Chain Quinoxaline Metallocyclophanes. J Org Chem 73(7): 2548-2553.
- Wipf P, Graham TH J (2004) Total Synthesis of (−)-Disorazole C1 Am Chem Soc 126(47): 15346-15347.
- Kokado K, Tokoro Y, Chujo Y (2009) Luminescent and Axially Chiral π-Conjugated Polymers Linked by Carboranes in the Main Chain Macromolecules 42(23): 9238-9242.
- Cheng H, Pu L (1999) Synthesis of chiral conjugated propellerlike polymers using optically active 1,1′‐binaphthyl‐2,2′‐diamine derivatives. Macromol Chem Phys 200(6): 1274-1283.
- Bédard AC, Vlassova A, Hernandez Perez AC, Bessette A, Hanan GS, et al. (2013) Chemistry A European Journal 19(48): 16295-16302.
- Bendikov M, Wudl F, Perepichka DF (2004) Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics. Chem Rev 104(11): 4891-4946.
- Shen Y, Chen CF (2012) Helicenes: synthesis and applications. Chem Rev 112(3): 1463-1535.






























