Microdroplet Chemistry: Difference of Organic Reactions between Bulk Solution and Aqueous Microdroplets
Soyul Kwak†, Jian Yeo†, Sungmin Jung†, Inho Nam*
Division of Chemistry & Bio-Environmental Sciences, Seoul Women’s University, Republic of Korea
†Equal contribution
Submission: May 14, 2018; Published: May 23, 2018
*Corresponding author: Inho Nam, Division of Chemistry & Bio-Environmental Sciences, Seoul Women’s University, Republic of Korea, Email: inhonam@swu.ac.kr
How to cite this article: Kwak S, Yeo J, Jung S, Nam I. Microdroplet Chemistry: Difference of Organic Reactions between Bulk Solution and Aqueous Microdroplets. Academ J Polym Sci. 2018; 1(1): 555551. DOI: 10.19080/AJOP.2018.01.555551
Abstract
Recent applications of microdroplet reactions are noted as reaction acceleration in confined environments and possible future scale-up synthesis compare to that of bulk phase reactions. Microdroplet reactions overcome the thermodynamic and kinetic challenges in an aqueous solution. Due to the microdroplet’s distinctive surface environment, the chemistry in Microdroplets significantly differs from that in the bulk solution.
Keywords: Microdroplet; Noncovalent complexations; Electrostatic potential; Organic reactions
Introduction
Remarkable findings intrigued people in different fields by showing that extremely slow bulk phase reactions can be accelerated dramatically in aqueous microdroplets [1,2]. Not only the rate of the reaction but also the yields of reaction increased due to negative free-energy change which is lower than the value in the bulk phase. Recent experiments have been done by several groups and they validated how microdroplet reactions enable to accelerate organic reactions such as addition reactions [3], condensation reactions [4,5], elimination reactions [2], substitution reactions [2], redox reactions [6], rearrangement reactions [7], and noncovalent complexations [8]. In this mini-review, we show the recent progress about the kinetic and thermodynamic change of organic reactions in aqueous microdroplets, as names as microdroplet chemistry, and its limitation.
To address how reactions in water microdroplets can significantly differ from those in the bulk phase, many experiments have been done and gave clues for the understanding (Table 1). In specific, the reduction of 2,6-dichlorophenolindophenol by the microdroplet fusion method is accelerated by a magnitude of 103 [6]. Base-catalyzed Claisen-Schmidt condensation of 1-indanone with electro spray ionization [ESI] is accelerated by a magnitude of 104 [4]. And the Pomeranz-Fritsch synthesis of isoquinoline, which is done by using ESI, is also accelerated by the order of 106 [7]. These reactions substantiate an increased rate in the microdroplet reaction by many orders of magnitude compared to the extremely slow kinetics in the bulk phase [1,2].

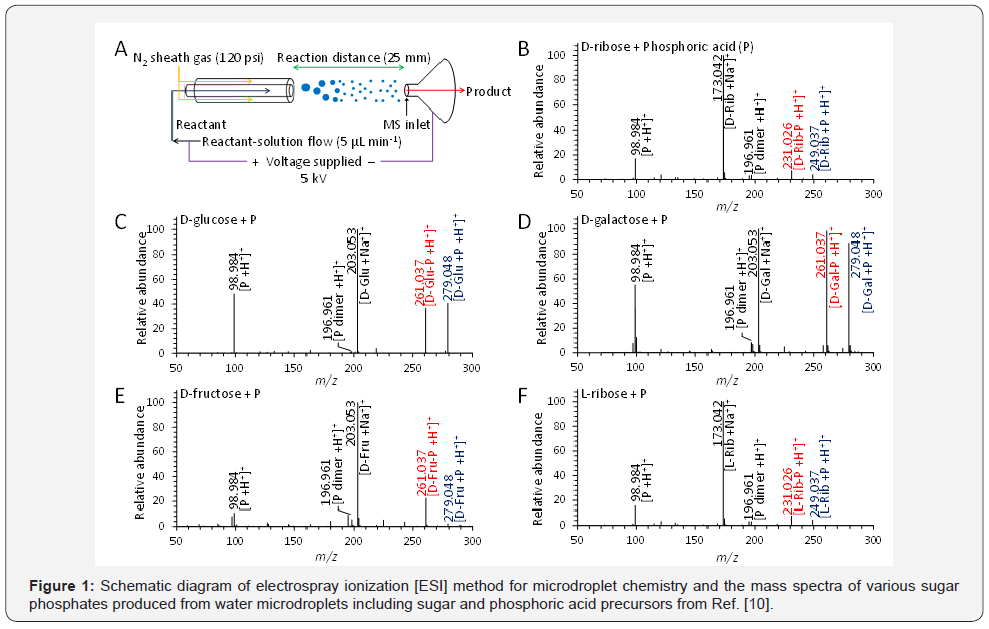
Within the distinctive environment of water microdroplets with different thermodynamic and kinetic properties compared to the bulk phase, an intriguingly plausible biochemical reaction mechanism in aqueous microdroplets were also reported, which might occur prebiotically in the absence of enzymes [9,10]. The abiotic production of sugar phosphates and ribonucleosides naturally does not occur in bulk aqueous solution, because of their thermodynamic hurdle [11]. Within a bulk phase, both phosphorylation and ribosylation of ribose, phosphate and nucleobases are extremely unfavorable, due to condensation of reagents accompanied by the elimination of water [12]. These reactions in bulk phase have an extremely low equilibrium constant [Keq] and a positive free-energy change, which leads to reverse process of hydrolysis of the biopolymer. On the contrary, the reaction in microdroplet provides a negative free-energy change. It substantiates a possible route for the prebiotically plausible formation of sugar phosphates and ribonucleo sides in microdroplets as shown in Figure 1. In the crowded interior of the microdroplets, longer range attractive forces can provide organization of molecules and decrease the organic reaction’s Gibbs free energy [13]. This leads favorable change in free energy and acceleration in reaction rate along with high yields. In consequence, although there is lack of evidence, the unique reaction environment by the surface of microdroplets might provide the clue of prebiotic chemical interaction which is significantly related to the possible routes of the origin of life [14]. Because, water-air interfaces on oceans, lakes, aerosols, and cloud and fog droplets can provide reaction environments for the abiotic organic reactions [15,16].
The organic reactions in water microdroplets [Microdroplet chemistry] are related to the concentration change of reactants in surface of microdroplets and the distinctive trait of the surface [1,2]. First, the concentration of reactants increases by the evaporation of a solvent on the surface of microdroplets, which makes a reaction acceleration [2]. In chemical reaction, Keq, is a value that never changes. To maintain that value, as the amount of reactants increases, the amount of the products must increase as well. Second, there is an orientation of reactants by the electrostatic potential on the surface of microdroplets, which brings about a decrease of an entropic hurdle in the chemical reaction [17]. At the interface, the characteristics of liqiudare different from in bulk solution. It means that the entropy of water in bulk is higher than at water-air interface and that there is very little chance that the water will react and form products. But, it is different when dealing with the water molecules at the air-liquid interface [9,10]. Near the interface, the water molecules organize and get anisotropic structures. This leads water molecules to gain charges and form an electric field, which affects the reaction rate [18]. That can make multiple parameters that affect the chemical reaction in the microdroplet such as pH, surface charge, reagent confinement, desolvation, contact ion pairing, large electrostatic pressure and molecular orientation on the droplet surface [9,10].
The above observations seem too common related to the kinetic and thermodynamic changes of organic reactions in water microdroplets. The superfast and thermodynamically changed organic reactions are maybe possible to open new chapter of chemistry [2]. At this chapter, we need to more precisely consider if there are some limitations in microdroplet chemistry. As shown before, many researches on acid-or basedcatalyzed reactions or the reactants containing polar functional groups proved that the reactions in the microdroplet are accelerated or thermodynamically changed [2-10]. However, there is little information about nonpolar compounds reaction in microdroplets. As a model of non polar organic reaction, the intra molecular Diels-Alder reaction of 3,5-hexadienyl acrylate ester was conducted recently [1]. The bulk phase, When the reaction in the bulk phase was performed with a catalyst of indium [III] triflate, it was reported that the reactants changed to Diels-Alder product at 70oC.When the reaction is conducted in microdroplets, the expected product is not observed, buthexa- 3,5-dien-1-ol was detected. It notes that the interface factor negligibly affects the nonpolar organic reaction because there is little chance of the special polar surface of microdroplets favor nonpolar reactive reagents [1].
Conclusion
We introduce the recent success of microdroplet chemistry in view of the acceleration and thermodynamic preference change of general organic reactions. The reaction mechanisms are not completely understood, there are two main factors for the favorable change of the kinetics and thermodynamics of reactions in microdroplets. Concentration is one factor that attributes to the acceleration of the reaction rate in confined aqueous environments [2]. Because of the surface area of the droplets increases, the area in which droplets can evaporate increases as well compared to that of the bulk solution. This leads to an increase in the concentration of the reactants. To balance the equilibrium, the products increase as the reactants increase, which leads to a higher amount of yields. Second, the natural high surface to volume ratio of microdroplets can generate unique surface characters at the air-water interface [1- 8]. As molecules in water droplets approach closer to interface between water and air, their organized water-air environment leads electric field to alter pH, surface charge, and orientation of reagents at surfaces [19,20]. Even though there is a limitation that water microdroplet chemistry is not favorable to nonpolar interaction, the water microdroplet chemistry can lead to facile organic synthesis technique and give the precise knowledge of chemistry in confined environment including biochemical processes in a cell nature [14].
Acknowledgment
This research was supported by a research grant from Seoul Women’s University (2018).
References
- Banerjee S, Gnanamani E, Yan X, Zare RN (2017) Can all bulk-phase reactions be accelerated in microdroplets? Analyst 142(9): 1399-1402.
- Yan X, Bain RM, Cooks RG (2016) Organic Reactions in Microdroplets: Reaction Acceleration Revealed by Mass Spectrometry. Angew Chem Int Ed 55(42): 12960-12972.
- Girod M, Moyano E, Campbell DI, Cooks RG (2011) Accelerated bimolecular reactions in microdroplets studied by desorption electrospray ionization mass spectrometry. Chem Sci 2: 501-510.
- Müller T, Tawiah AB, Cooks RG (2012) Accelerated Carbon-Carbon Bond‐Forming Reactions in Preparative Electrospray. Angew Chem Int Ed 51(47): 11832-11835.
- Bain RM, Pulliam CJ, Cooks RG (2015) Accelerated Hantzsch electrospray synthesis with temporal control of reaction intermediates. Chem Sci 6: 397-401.
- Lee JK, Kim S, Nam HG, Zare RN (2015) Microdroplet fusion mass spectrometry for fast reaction kinetics. Proc Natl Acd Sci USA 112(13): 3898-3903.
- Banerjee S, Zare RN (2015) Cover Picture: Velocity of a Molecule Evaporated from a Water Nanodroplet: Maxwell–Boltzmann Statistics versus Non‐Ergodic Events. Angew Chem Int Ed 54: 14795-14799.
- Lee JK, Banerjee S, Nam HG, Zare RN (2015) Acceleration of reaction in charged microdroplets. Rev Biophys 48: 437-444.
- Nam I, Nam HG, Zare RN (2018) Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets. Proc Natl Acd Sci USA 115(1): 36-40.
- Nam I, Lee JK, Nam HG, Zare RN (2017) Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets. Proc Natl Acd Sci USA 114(47): 12396-12400
- Xu J et al. (2017) A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization. Nat Chem 9: 303-309.
- Powner MW, Gerland B, Sutherland JD (2009) Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459: 239-242
- Takats Z, Nanita SC, Cooks RG (2003) Serine Octamer Reactions: Indicators of Prebiotic Relevance. Angew. Chem Int Ed 42: 3521-3523.
- Vaida V (2017) Prebiotic phosphorylation enabled by microdroplets. Proc. Natl. Acd. Sci. USA 114(47): 12359-12361.
- Dobson DM, Ellison GB, Tuck AF, Vaida V (2000) Atmospheric aerosols as prebiotic chemical reactors. Proc Natl Acd Sci USA 97(22): 11864- 11868.
- Griffith EC, Tuck AF, Vaida V (2012) Ocean–Atmosphere Interactions in the Emergence of Complexity in Simple Chemical Systems. Acc Chem Res 45(12): 2106-2113.
- Fallah-Araghi A, Kamel M, Thomas M, Martin K, Jean-Christophe B, et al. (2014) Enhanced Chemical Synthesis at Soft Interfaces: A Universal Reaction-Adsorption Mechanism in Microcompartments. Phys Rev Lett 112, 028301.
- Kathmann SM, Kuo IFW, Mundy CJ, Mundy J (2008) Electronic Effects on the Surface Potential at the Vapor−Liquid Interface of Water Am Chem Soc 130(49): 16556-16561
- Donaldson DJ, Vaida V (2006) The Influence of Organic Films at the Air-Aqueous Boundary on Atmospheric Processes. Chem Rev 106: 1445-1461
- Tervahattu H, Juhanoja J, Vaida V, Tuck AF, Niemi JV, et al. (2005) Fatty acids on continental sulfate aerosol particles. J Geophys Res 110, D06207.






























